Crotalus lepidus lepidus Venom Produces Hemolysis and Lipid Peroxidation in Human Erythrocytes in vitro
Author: Alejandro Zugasti-Cruz
Abstract
The hemolytic and lipid peroxidative effects of crude venom from Crotalus lepidus lepidus, a rattle snake found in the Northeast of Mexico and southeast of the United States, were investigated in human erythrocytes in vitro.
Significant concentration-dependent effects were found on both
hemolysis (evaluated as release of hemoglobin) and lipid peroxidation
(as a common index of oxidative damage to membrane lipids) in the red
blood cells. These results suggest that hemolysis was produced with the
involvement of oxidative stress as a potential mechanism of toxicity in
the erythrocytes.
Keywords: Hemolysis; Lipid Peroxidation; Crotalus lepidus lepidus Venom
Abbreviations: TBA: Thiobarbituric Acid; TCA: Trichloroacetic Acid; MDA: Malondyaldehide; (C.l. lepidus): Crotalus Lepidus Lepidus
Introduction
Snake venoms are among the best pharmacologically
characterized natural toxins, chiefly because of their deleterious
effects on humans [1].
Snake venoms are toxic secretions, which are complex mixtures of
molecules of different biochemical nature, with a predominance of
proteins, many of which are endowed with enzymatic activities [2].
The most common snake venom enzymes include acetylcholinesterases,
L-amino acid oxidases, serine proteinases, metalloproteinases and
phospholipases A2 [2]. Some phospholipases A2
cause a wide variety of pharmacological effects such as pre-synaptic or
post- synaptic neurotoxicity, miotoxicity, anticoagulant and hemolytic
activities [3,4]. PLA2 catalyzes the specific hydrolysis of ester bonds at the C2 position of 1,2-diacyl-3-sn-glycerophospholipids with release of free fatty acids. Thus, phosholipases A2 are able to disrupt the phospholipid packings from several types of biological membranes, leading to cell lysis [5]. There is some evidence that animal venoms can induce oxidative stress. For example, El Asmar et al. and Dousset et al. [6,7]
have reported that the increase in polyunsaturated fatty acids
following envenomation by scorpion venom may lead to an increase in the
rate of lipid peroxidation, which might be responsible for tissue
damage. In the case of snakes, the venom of Echis piramidum causes lipid peroxidation in different organs of mice [8] and the venom of Bothrops induces renal tubular toxicity mediated in part by lipid peroxidation [9].
Currently, lipid peroxidation is one of the most important organic
expressions of oxidative stress induced by the free radicals produced
during various types of xenobiotic exposures or pathological conditions,
animal venoms included. The potential consequences of the peroxidative
process of membrane lipids include loss of polyunsaturated fatty acids,
decreased lipid fluidity, altered membrane permeability, effects on
membrane-associated enzymes, altered ion transport, release of material
from subcellular compartments, and the generation of cytotoxic
metabolites or lipid hydroperoxides [10].
Furthermore, vertebrate red blood cell membranes have a high content of
unsaturated lipids as well as iron in hemoglobin, one of the most
powerful catalysts capable of initiating lipid peroxidation [11].
In addition, erythrocytes are anucleated cells and therefore lack
protein synthetic machinery, they cannot replace many cellular
components, so oxidative damage may induce a permanent alteration in the
red cells [11]. In this study, we evaluated hemolytic response and lipid peroxidation of the crude venom from the rattlesnake Crotalus lepidus lepidus on isolated human erythrocytes.
Materials and Methods
Chemicals
Sodium Citrate, Anhydrous Dextrose, Sodium Chloride,
Citric Acid, Thiobarbituric Acid (TBA), Trichloroacetic Acid (TCA) were
all purchased from J.T. Baker (Center Valley, PA). All other reagents
were from Sigma (St. Louis, MO).
Preparation of venom extract
The venom of Crotalus lepidus lepidus was
purchased from the laboratory of National Natural Toxins Research Center
at Texas A&M University-Kingsville. Venom solution was prepared
using 10 mg of lyophilized venom in 5.0 mL of Alsever's solution pH 6.4
(dextrose 0.116 M, NaCl 0.071 M, sodium citrate 0.027 M, and citric acid
0.002 M), then, centrifuged at 12,000 g for 10 min at 4°C. The pellet
was discarded and the supernatant was aliquoted and stored at -20°C
until use. Protein concentrations were determined in samples by the
method of Bradford (1976) [12] with bovine serum albumin as a standard.
In vitro hemolysis assay
The present study was approved by the Ethics
Committee on Animal Experimentation of the Faculty of Chemistry of the
Autonomous University of Coahuila, Mexico. The hemolysis test was
performed using human whole blood from healthy non-smoking donors with
permission, following guidelines for studies using human samples. In
briefly, blood was collected in heparinized-tubes, was centrifuged at
3000 rpm for 4 minutes at 4°C. The pellet was washed three times with
cold Alsever's solution. The supernatant was then removed and 100 mL of
the purified erythrocytes were diluted 1: 99 with Alsever's solution.
Then, 150 mL of this suspension was suspended in Alsever's buffer and
taken for the curve-response experiments (total volume 1500 ml). This
suspension of red blood cells was always freshly prepared and used
within 24h after collection. Crude venom concentrations were tested:
0.250, 0.500, 1.0 and 2.0 mg/mL. The tubes were gently mixed in a
rotator shaker, and then incubated at 36.5°C ± 1°C within a shaking
water bath, for 60 minutes. Alsever’s solution and deionized water were
used as negative and positive controls, respectively. Each group
contained three tubes. The samples were then centrifuged under 3000 rpm
for 4 minutes to collect the supernatant. The absorbance (A) value of
the hemoglobin released from the erythrocyte cells was measured
spectrophotometrically at 415 nm (Thermo Spectronic Genesys 5). All
trials were run three times. The absorbance obtained was correlated with
a curve of human hemoglobin.
The experiments were run in triplicate and were repeated twice.
Determination of malondialdehyde (MDA)
Erythrocytes MDA concentration was determined by
using the method described by Draper and Hadley (1990) based on TBA
reactivity (TBARs) with some modifications. In this manner, 1.0 mL of
supernatant of the erythrocytes suspension (it was prepared in the
similar way as the hemolytic assay) was mixed with 45 mL of TCA (50%).
The samples were centrifugated at 3000 rpm/10 min. Then, 115 mL of TBA
(0.75 % in 0.1 M HCl) was added and put into a boiling water bath for 10
min. Tubes were centrifuged at 3,000 rpm for 10 min, and the absorbance
was measured at 535 nm. Results were expressed as nmol TBARs/ mg
protein. Concentration of MDA was calculated by a calibration curve
using 1,1,3,3-tetraethoxypropane as a standard.
Phospholipase A2 activity assay
The method of Araujo and Radvanyi [13]
was used with some modifications. Venom solution (10 mg in 10 ml of PBS
pH 7.4) was added to the reaction medium (1.5 mL) containing 15 mol
phosphatydylcholine, 18 mol Triton X-100, 5mol calcium chloride, 80 mol
phenol red and 7.5 mmol Tris at pH 7.9. Subsequently, the solution was
incubated for 5 min at room temperature and added to a cuvette. Changes
in the absorbance at 558 nm were followed during 5-8 min. Decrease in
absorbance of phenol red by the acidification of medium was proportional
to the liberation of fatty acids. The specific activity was calculated
in Units (mM) of fatty acid per min. Controls included samples incubated
with Alsever solution instead of venom. This assay provides information
about presence and activity of phospholipases A2 in the venom of C. I. lepidus.
Statistical Analysis
All data are expressed as mean SEM. All experiments
were performed in triplicate and three independent experiments were
carried out. For the statistical analysis of results, we employed
one-way analysis of variance (ANOVA) followed by Dunnett's test for
specific comparisons against control values. Values of p<0.05 was
accepted as significant levels.
Results
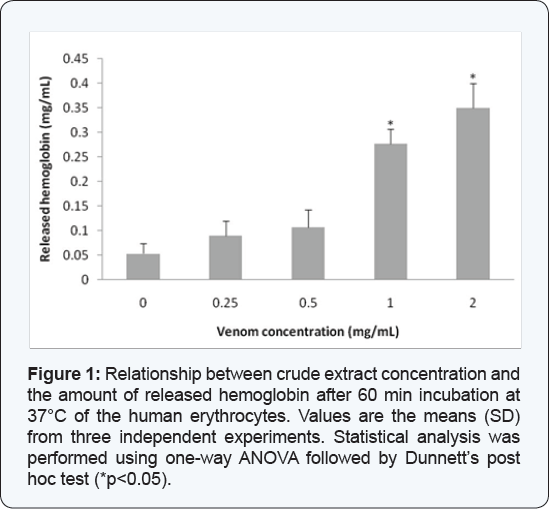
The crude extract of Crotalus lepidus lepidus
added to the red blood cells resulted in a concentration-dependent
hemolytic response, expressed as percentage of released hemoglobin,
obtaining the following values: 0 mg/mL (0.05 mg Hb/mL), 0.25 mg/mL
(0.09 mg Hb/mL), 0.5 mg/mL (0.106 mg Hb/mL), 1 mg/ mL (0.276 mg Hb/mL)
and 2 mg/mL(0.35 mg Hb/mL) (Figure 1).
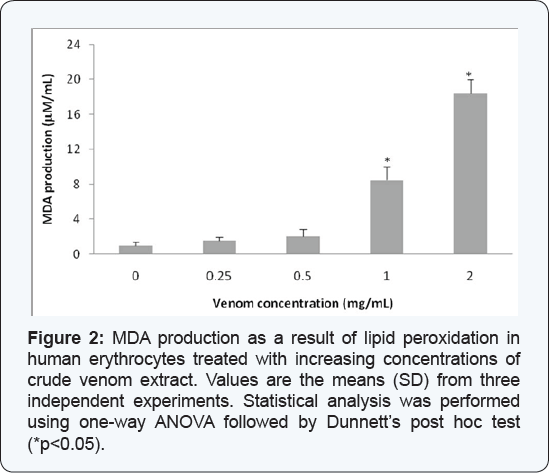
Lipid peroxidation in erythrocytes
Significant increases in the levels of peroxidized
products (measured as MDA production), with respect to control group,
were found in the blood cells exposed to concentrations of the crude
venom of C. l. lepidus of 1.0 mg/mL (8.5 mM/mL) and 2.0 mg/mL (20.12 mM/mL) (Figure 2).
Phospholipase A2 activity
This assay was made to investigate presence and activity of phospholipases A2 in the venom of C. l. lepidus. The phospholipase A2 activity of the venom of C.l. lepidus
(1mg/mL) was 0.041±0.0035 (mM/min). This concentration was chosen
because it provoked the first increase in hemolysis and MDA production
assays.
Discussion
The mottled rock rattlesnake (Crotalus lepidus lepidus) is one of four subspecies of C. lepidus.
It is a relatively small rattle snake non aggressive although exhibits a
wide range distribution includes southwest Texas, southeastern New
Mexico and the Mexican Plateau to San Luis Potosi [14]. In venom of C.l. lepidus has been identified some partial sequences of metalloproteinases, galactose-specific lectins and phospholipases A2, as well produces hemorrhagic and lethal activities in mice [15]. The clinical effects of a case envenomation by C.l. lepidus include hemorrhagic effects, ecchymosis, soft tissue swelling, pain, thrombocytopenia and other hematological alterations [16]. Our results indicate that crude venom of C.l. lepidus in concentrations of 1.0 and 2.0 mg/mL is effective at producing toxicity in human erythrocytes in vitro,
as indicated by hemolysis and lipid peroxidation. We employed the
hemolytic response as a suitable tool to evidence cytotoxicity since
some authors have reported that hemolysis represents a very sensitive
test to assess and characterize phospholipases of snake venom [17,18] and the TBARs assays as a common index of lipid peroxidation [19,20] to assess the involvement of free radical formation during the cytotoxicity elicited by the crude venom of C.l. lepidus. In addition, we determined that C.l. lepidus venom possesses PLA2 activity, similar to the other Crotalus lepidus subspecies [15]. PLA2 enzymes are unique calcium-dependent hydrolytic enzymes on phospholipids, liberating free fatty acid and lysophospholipid [5,21]. Phospholipid hydrolysis by PLA2
enzyme also releases arachidonic acid whose metabolism results in the
formation of potentially toxic reactive oxygen species and lipid
peroxides. Red blood cells are particularly vulnerable to the attack by
free radicals and therefore, they represent a suitable substrate to give
evidence of oxidative stress [11,22]. Our results indicate that the venom of C.l. lepidus
induces lipid peroxidation in human red blood cells. Erythrocytes lysis
may be the end result of defects in the red blood cell membrane related
to the peroxidative attack probably mediated by PLA2,
suggesting that the peroxidative actions might, at least partially,
contribute to the hemolytic effect. Interestingly, Norris [16] reported a clinical case of a bite in a person of 30 years by a captive specimen of C.l. lepidus
who provokes a reduction in hematocrit and hemoglobin values in the
patient. Erythrocytes are also susceptible to oxidative stress due to
unsaturated membrane phospholipids, and the presence of hemoglobin and
other hematin compounds may also augment the process of lipid
peroxidation [23].
In addition, the erythrocytes have a high content of iron in
hemoglobin, a powerful catalyst capable of initiating lipid peroxidation
[11].
It's well known that lipid peroxidation produces oxidation of lipids,
particularly polyunsaturated fatty acids and cholesterol [22,23].
In the lights of these results, the hemolytic and lipid peroxidation
response to the crude venom was concentration- dependent, suggesting
that part of the toxic action of the venom corresponds to the formation
of free radicals mediated by PLA2, and hence the consequent changes in membrane structure after lipid peroxidation.
Conclusion
The findings of our study indicate that the crude venom from C.I. lepidus is able to induce hemolysis and lipid peroxidation in the isolated human red blood cells.
Acknowledgement
The financial grant was provided by the Secretaria de
Educacion Publica, Subsecretaria de Mejoramiento del Profesorado,
Programa de Mejoramiento del Profesorado. Mexico.
Conflict of Interest
Conflicts of Interest: There is no conflict.
For more
details Open Access Journal of Toxicology (OAJT) please
click on: https://juniperpublishers.com/oajt/index.php
To read more…Full Text
in in Juniper Publishers click on https://juniperpublishers.com/oajt/OAJT.MS.ID.555554.php

Comments
Post a Comment