Evaluation of Chronic Oral Nicotine Treatment in Food Consumption, Body Weight and [125I] Epibatidine Binding in Adult Mice
Authored by Ursula H Winzer-Serhan
Abstract
Despite its abuse potential, nicotine, acting on
nicotinic acetylcholine receptor, has possible medicinal uses, in
particular in treating neuro degenerative diseases. Therefore, animal
models to evaluate exposure need to be characterized. Administration via
drinking water is a stressfree route of administration but often
results in low blood nicotine levels. Here we evaluated chronic exposure
to low, medium and high concentrations of nicotine in drinking water.
Three-month-old C57BL/6 male mice were treated for 23 days with 20, 120
or 300 ^g/ml nicotine in 2% saccharin water, corresponding to 5, 30 and
55 mg/kg/d, respectively. Food intake and body weight were monitored,
blood nicotine and cotinine levels, and 125I-epibatidine-binding sites
were determined at day 23.Average blood cotinine levels of 11.7, 151.8
and 192.0 ng/ ml were detected in mice receiving the low, medium and
high dose, respectively. In contrast, nicotine was only consistently
measured in the group receiving 300 μg/ml, with an average blood level
of 6.2 ng/ml and was the only treatment group to exhibit significantly
decreased food intake (p=0.005) and body weight (p=0.043), as well as
increased I125-epibatidine binding in cortex (p=0.055) and hippocampus
(p = 0.019). We evaluated possible effects of chronic nicotine
(300^g/ml) exposure on anxiety-like behavior using the open field test.
An anxiolytic effect was found compared to controls and there was no
evidence for anxiogenic effects of chronic nicotine. Thus, a high
concentration of nicotine in drinking water was necessary to achieve
consistent blood nicotine levels in mice, which correlated with markers
considered hallmarks of chronic nicotine treatment.
Keywords: Nicotine; nAChR; Hippocampus; Receptor Binding; Body Weight; Food Intake; Anxiety
Abbreviations:
AD: Alzheimer's Disease; ANOVA: Analysis of Variance; DG: Dentate
Gyrus; nAChR: Nicotinic Acetylcholine Receptor; ROD: Raw Optical
Density.
Introduction
The plant alkaloid nicotine interacts with neuronal
nicotinic acetylcholine receptors (nAChRs), which are pentameric ligand
gated cation channels that are either homomerically or heteromerically
formed by different subunit combinations. Among the possible subunit
combinations, heteromeric α4β2 nAChRs are the most widely expressed
heteromeric nAChR subtype in the mammalian brain and exhibit high
affinity for nicotine [1,2]. Nicotine, the major psychoactive ingredient in tobacco smoke [3], facilitates dopamine release and activates the reward pathway [4,5], and as such is considered an addictive drug, and strongly implicated in tobacco dependence [6,7].
However, nicotine may also have beneficial effects; the drug has shown
cognitive enhancing, anxiolytic and neuro protective properties in
animals and humans [8-10], may slow age-related neuronal decline, and reduce neuronal degeneration in Alzheimer's or Parkinson's disease [11,12].
Neuro protective effects of nicotine are the result of nAChR
activation; in particular, activation of heteromeric nAChRs seems to be
important. Transgenic mice that lack the β2 subunit (β2 nAChR knockout
(KO) mice) and thus do not express high affinity nAChRs, have reduced
adult neurogenesis [13]
and exhibit accelerated aging in cortical areas, underscoring the
importance of heteromeric nAChRs to maintain a healthy mature brain [14,15].
Thus, medicinal use of nicotine or related nAChR agonists could have
great beneficial effects for human health. Therefore, despite the known
potential for abuse, nicotine and nicotinic receptor agonists are being
evaluated for therapeutic uses [16-18].
Medicinal use of nicotine would often require long-term drug
administration. To study effects of nicotine administration in adult
animal models over several weeks or month, the route of nicotine
administration becomes very important. Voluntary oral consumption of
nicotine via drinking water is non-invasive, stress-free and requires no
additional animal handling. Therefore, this route of drug is suitable
for long-term treatment in preclinical animal models, and is a viable
alternative to daily injections or use of osmotic mini pumps. In
addition, administration via drinking water results in nicotine
fluctuations during a 24-hour period, which allows time for
re-sensitization of desensitized nAChRs. On the other hand, oral
nicotine administration results in poor bioavailability, which means
that the fraction of administered nicotine reaching systemic circulation
is lower compared to other routes, and results in low and more variable
levels of nicotine in the brain. This is due to ion trapping of
nicotine in the acidic milieu in the stomach [19], slow absorption through the gastrointestinal tract, and rapid first pass metabolism in the liver [20,21]. In mice in particular, nicotine is rapidly metabolized, and strain specific metabolic rates for nicotine may differ [22,23].On the other hand, nicotine can be absorbed directly through the buccal cavity where first pass metabolism is not a factor [19].
Together, these properties make it difficult to estimate effective
doses of orally administered nicotine based solely on dose [24,25].
Two hallmarks of chronic nicotine exposure have been described for
decades: an anorexic effect that results in decreased food consumption
and body weight [26-29], and an increase in high affinity nAChR binding sites [30-33].
Together with determining blood nicotine and cotinine levels, we used
these two hallmarks to evaluate central effects of chronic oral nicotine
administration in adult C57BL/6 mice. In addition, there are reports
that chronic nicotine exposure affects anxiety in humans and in animal
models [34-38]. Therefore, we also evaluated the effects of chronic nicotine administration on anxiety-like behavior.
Materials and Methods
Animals and Drug Administration
Young adult male C57BL/6 mice were obtained from a
breeding colony established at Texas A&M University's Laboratory
Animal Research and Resource facility from mice originally obtained from
the Jackson Laboratory, Bar Harbor, ME. At three months of age, mice
were individually caged and randomly assigned to different dosing groups
(n = 5). Then all mice received 2% saccharin (saccharin sodium salt
hydrate, >98%) (Sigma, St. Louis, MO, USA) in their drinking water
starting two weeks prior to actual dosing with nicotine to establish
their individual daily drinking volume. (-) -Nicotine (hydrogen
tartrate) (Sigma) was administered in 2% saccharin containing water.
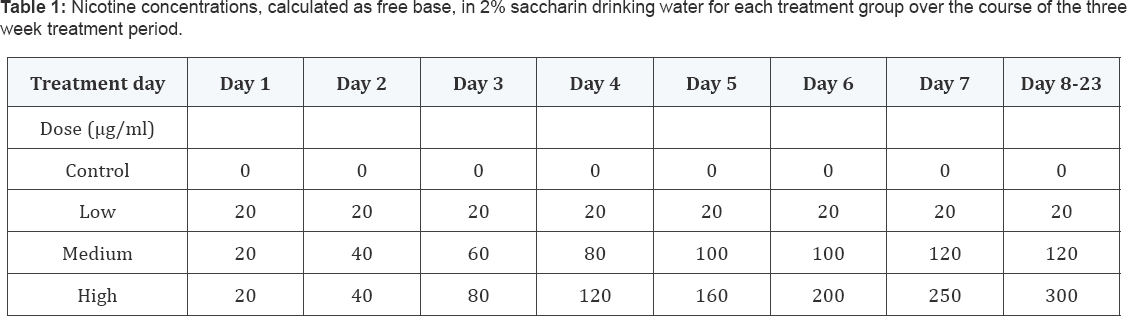
Four nicotine concentrations (given as free base) were tested: control -
0 μg/ml (0 mg/kg/day), low dose(LD) - 20 μg/ ml, medium dose (MD)- 120
μg/ml and high dose (HD)- 300 μg/ml resulting in approximately 5, 30 and
55 mg/kg/day free base nicotine, respectively, calculated based on
average liquid consumption and body weight. Animals in the MD-and HD-
groups initially received 20 μg/ml of nicotine on the first day of
treatment and the concentrations were gradually increased until the
final concentrations were reached on day 7 and day 8 of treatment,
respectively (Table 1).
Individual water consumption was not significantly affected until a
concentration of 200 μg/ ml was reached; at 300 μg/ml nicotine, water
intake was 86.1% (77.7 - 98.08 %) of the initial individual consumption
levels (p=0.018). All mice were treated until day 23. Access to food and
drinking solutions was ad libitum. Food intake and body weight were
assessed every three days, starting from day 1 to day 19, and normalized
to the first measurement of each individual animal. On days 20 to 22,
the mice were subjected to behavioral testing, and then euthanized on
day 23. All procedures were approved by the Texas A&M University
Animal Use Committee and carried out in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory Animals
(National Institutes of Health Publication No. 85-23, revised 1996).
Open Field Test
To test locomotor, exploratory and anxiety behavior,
on day 20 of nicotine treatment, mice were placed individually in an
open field (length x width x height: 20 x 20 x 30 cm) (Versa max animal
activity monitor and analyzer, AccuScan Instruments, Columbus, OH, USA)
equipped with vertical and horizontal infrared beam sensors. The
apparatus was constructed using transparent Plexiglas, located in a
quiet room, and environmental cues including lighting and experimenter
were kept constant during testing. Mice were acclimated to the
experimental room for 5 minutes, and then placed in the open field for
30 minutes. Activitywas recorded every minute. The open field chamber
was cleaned with 50% ethanol between mice. Horizontal activity, total
distance, movement time, rest time, stereotype counts, stereotype time,
margin distance, margin time, center distance, center time, rearing
activity, and rearing time were automatically recorded.
Tissue preparation
All mice were anesthetized using isoflurane (IsoFlo,
North Chicago, IL, USA) and decapitated on day 23 in the morning.
Approximately 1 ml of trunk blood was collected from each mouse, kept on
ice, and centrifuged at 4°C at 3000 rpm for 20 minutes. One hundred to
300 μl of serum was collected from each mouse and sent to Bio analytical
Core Laboratory Service Center (Department of Pharmaceutics, School of
Pharmacy, Virginia Commonwealth University, Richmond, VA, USA) for
analysis of nicotine and cotinine levels. The brains were rapidly
dissected, frozen on powdered dry ice, and stored at -80°C.
[125I]-Epibatidine Binding
Radioactive, iodinated [125I]-epibatidine ligand was
used to evaluate the relative levels of heteromeric nAChR binding sites.
Receptor autoradiography was performed as previously described [39];
briefly, tissue sections were warmed to room temperature (RT) and
pre-incubated 5 minutes in Tris-HCl buffer (50 mMTris-HCl, 120 mMNaCl, 5
mMKCl, 2.5 mM CaCl2, 1 mM MgCl2, pH 7.4). Then sections were incubated
with 0.4 nM [125I]-epibatidine (PerkinElmer Life Science NEX358, Boston,
MA, USA, specific activity: 2200 Ci/mmol) for 1 hour at RT. For
non-specific binding, sections were incubated with the presence of 400
μM (-)-nicotine hydrogen tartrate. Sections were washed in ice-cold
Tris-HCl buffer twice for 1 minute each, followed by 10 seconds in cold
ddH2O, and dried under air stream for 1 hour Sections were dried at RT
over night before exposure to BioMax MR Film (Kodak, Rochester, NY,
USA). After 1-day of exposure, films were developed in D19 Kodak
developer for 4 minutes, rinsed in water, fixed in Kodak Rapid Fixer for
5 minutes, and air-dried.
Data Analyses
Quantitative analysis of auto radiograms was done
using a PC-based image analysis system, MCID basic (InterFocus Imaging
Ltd, Haverhill, Suffolk, UK). Receptor binding levels were measured as
raw optical density (ROD) in the hippocampus (combined measures in the
molecular layer of the dentate gyrus (DG) and the stratum lacunosum
molecular of the CA1 in hippocampus proper), thalamus (the post thalamic
nucleus and the ventral posteriomedial thalamic nucleus), and cerebral
cortex, specifically layers IV-V of the visual and somato sensory
cortex. All anatomical structures were determined according to the Mouse
Brain Library atlas. SPSS 14.0 was used for statistical analysis.
Significance was defined as p ≤ 0.05. For food intake and body weight
changes, repeated measurements were used for between-subject effects
after nicotine reached the highest dose in all groups (day 8), and
Student's t-tests were used to determine significant differences at each
time point between nicotine and control groups. For receptor binding,
one-way analysis of variance (ANOVA) was used and Tukey HSD was
performed as a post hoc test when needed.For open field test, Student's
t-test was used to compare nicotine and control groups. All data are
presented as average ± standard error the mean (SEM).
Results
Blood Nicotine and Cotinine Levels
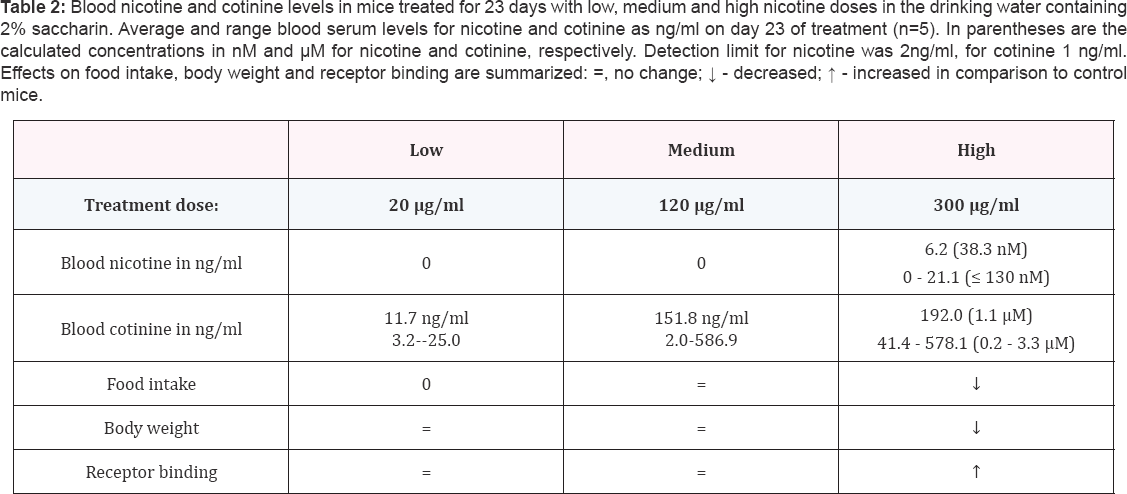
Nicotine or cotinine were not detected in the serum
of control mice (< 2 ng/ml and < 1 ng/ml, respectively).In the
LD-and MD- group,4 out of 5 mice had undetectable levels of nicotine
(< 2 ng/ml) in blood serum, with one mouse in the LD group with 2.9
ng/ml and one mouse in the MD group with 19.7 ng/ml nicotine. Cotinine
levels ranged from of 3.2 to 25.0 ng/ml with an average of 11.7 ng/ml in
the LD group (20 μg/ ml), and from 2.0 to 586.9 ng/ml with average of
151.8 ng/ml in the MD group (120 μg/ml) (Table 2).
In the HD group (300 μg/ml) four out of five mice had detectable blood
nicotine levels ranging from 2.1 to 21.1 ng/ml with an average of
6.2ng/ml and cotinine concentrations had a range of 41.4 to 578.1 ng/ ml
and an average of 192 ng/ml. There were no differences in food intake
and body weight between mice in the control, LD- and MD-groups (food
intake: repeated measurements, between- subject effect, p = 0.52; body
weight: repeated measurements, between-subject effect, p=0.88). In
addition, numbers of nACh Rreceptor binding sites detected with receptor
autoradiography using 125I-epibatidine binding, showed no significant
difference between brain sections derived from mice in the control
versus LD-or MD-groups in cortex, hippocampus or thalamus (one-way
ANOVA; cortex:p = 0.63; hippocampus: p = 0.56; thalamus: p =0.45). Thus,
low and medium doses of nicotine administered via drinking water, did
not result in consistent blood nicotine levels and did not affect food
intake, body weight or nicotinic receptor binding (Table 2).
Only the highest dose of nicotine tested in this study resulted in
significant differences in these measures, which we will describe in
more detail.
Food Intake and Body Weight
Food intake at the first measurement time point was
similar in control- and HD-groups (control: 3.61 ± 0.14 g/day; HD-
group: 3.75 ± 0.07 g/day). Subsequent measurements were normalized to
the first measurement for each individual mouse, and comparisons were
made between groups starting from day 8, when the high dose of 300μg/ml
nicotine in the drinking water was reached (Table 1).
After day 8, the average daily food intake was significantly lower in
mice in the HD-group compared to control-group (control: 4.30 ± 0.26
g/day; HD-group: 3.79 ± 0.13 g/day; repeated measurements,
between-subject effect, p=0.005) (Figure 1A).
Overall, mice in the HD-group decreased their food intake by about 12%
compared to control mice. Body weights on the first day of treatment
were identical between groups (control: 26.59± 1.00 g; HD-group: 26.39 ±
0.70 g), and subsequent measurements were normalized to the initial
weight of each individual animal. Body weights in control mice increased
continuously, over the treatment period and mice gained, on average,
0.16 ± 0.04 g/day (Figure 1B).
In contrast, after day 8, when the full-dose of nicotine was reached in
the HD-group, mice exhibited a slight loss in body weight of 0.02 ±
0.07 g/day. This resulted in increased body weight in control mice but
not in mice from the HD-group on day 19 compared to their individual
weight on day 1 (Student's t-test, control: p=0.02; nicotine: p=0.814),
and subsequently a 4% decrease in body weight between treatment groups
on day 19 (control: 27.59 ± 0.83 g; HD-group: 26.39 ± 0.51 g; body
weight, repeated measurements, between-subject effect, p = 0.043).

Nicotinic receptor autoradiography
Nicotinic receptor autoradiography using
125I-epibatidine, was performed to evaluate nicotinic receptor binding
sites after chronic treatment to validate nicotine exposure in the brain
[40].
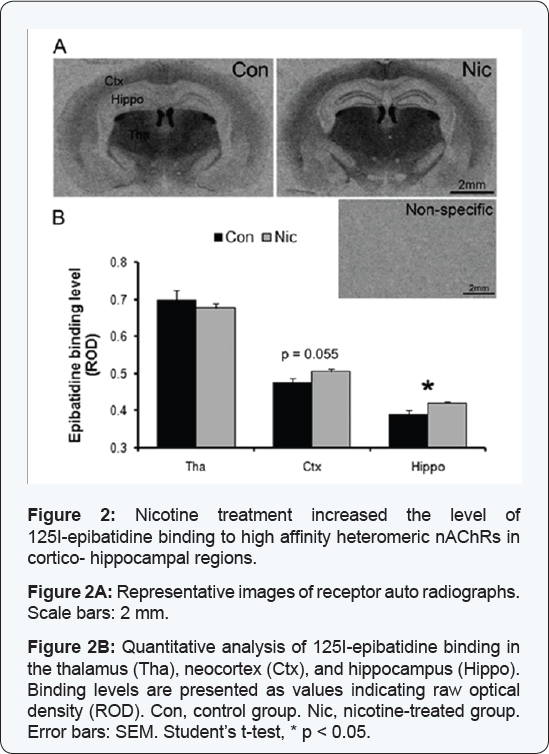
Non-specific binding was negligible, and the specific binding pattern
was similar to those previously reported with strong binding in the
thalamus, medial habenula, and fasciculus retroflexus, moderate binding
in cortex and low levels of binding in the hippocampus [41,42] (Figure 2A).
In the hippocampus, relative 125I-epibatidine binding, measured in
stratum lacunosum molecular of the CA1 and in the molecular layer of the
dentate gyrus, was significantly higher in brain slices from mice in
the HD-group compared to control mice (ROD control: 0.39 ± 0.010;
nicotine: 0.42 ± 0.003; Student's t-test, p = 0.019) (Figure 2B).
In the cortex, relative 125I-epibatidine binding level was slightly
higher in mice from the HD-group compared to control mice (control: 0.48
± 0.011; nicotine: 0.50 ± 0.007; Student's t-test, p = 0.055) (Figure 2B).
In contrast, in the thalamus, relative 125I-epibatidine binding was not
affected by high dose nicotine treatment (control: 0.70 ± 0.024;
HD-group: 0.68 ± 0.011, p = 0.435) (Figure 2B).
Effects on anxiety like behavior
Nicotine, when used for medicinal purposes, should
not have anxiogenic effects. Therefore, we used the open field test to
evaluate anxiety-like behavior in mice from control- and HD- group. On
day 20 of treatment, locomotor activity, exploratory- and anxiety-like
behaviors were assessed in the open field test. Of the 12 parameters
measured (see material and method section), 10 parameters showed no
significant differences (Student’s t-test, p >0.05) indicating that
locomotor activity and exploratory behaviors were neither impaired nor
augmented in the HD-group compared to control. Only margin and center
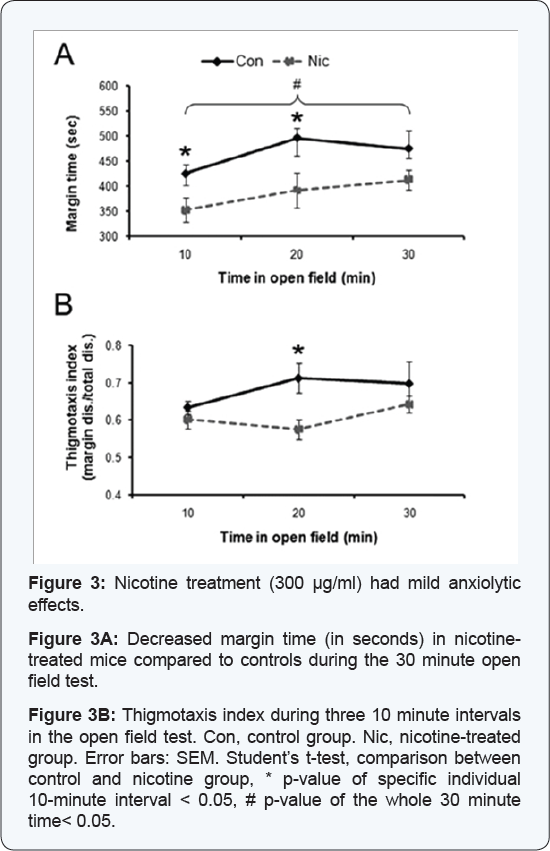
time, two parameters that indicate anxiety levels, were significantly
affected. Control mice spent 78% of the time at the margins,
significantly longer than mice from the HD-group, which spent only 64%
of the time at the margins (30 minutes overall, Student’s t-test, p =
0.025) (Figure 3A).
When activity was analyzed in 10 minute intervals, control mice spent
significantly more time in the margin area compared to mice from the
HD-group during the first and second 10 minute intervals (first
interval: control: 425 ± 17 sec, HD-group: 352 ± 24 sec, Student’s
t-test, p = 0.037; second interval: control: 496 ± 19 sec, HD-group: 392
± 35 sec, Student’s t-test, p = 0.031). Group differences diminished
during the last ten minutes when mice in the HD-group increased the time
spent at the chamber’s margins (control: 475 ± 36 sec; HD- group: 411 ±
20 sec) (Figure 3A).
These results suggested that chronic nicotine had no anxiogenic
effects, but to further assess this possibility, we calculated the
thigmotaxis index (margin distance / total distance), which has been
suggested to bean index of anxiety in mice [43].
The thigmotaxis index was slightly higher in control mice compared to
mice from the HD-group, with significant differences during the second
10 minute interval (control: 0.71 ± 0.04; HD-group: 0.59 ± 0.03;
Student’s t-test, p = 0.021) (Figure 3B), which also suggested reduced anxiety in mice treated with the high dose of nicotine.
Discussion
The goal of this study was to determine an oral dose
of nicotine that is well tolerated by mice, and induces central effects
typically associated with chronic nicotine. In this study we show that
voluntary oral consumption of nicotine-containing drinking water can
result insignificant decreases in food intake and subsequently body
weight, and increased nicotinic receptor binding, measures which are
considered hallmarks of chronic nicotine exposure. However, these
effects were only seen with the highest dose of nicotine (300 μg/ml)
administered in the drinking water, which was also the only dose that
resulted consistently in measurable blood nicotine levels in the adult
male mice used in this study. We used three different concentrations of
nicotine in the drinking water (20, 120 and 300 μg/ml) and measured the
resulting blood nicotine and cotinine levels after three weeks of
exposure. All three doses were well tolerated by the mice with no
obvious negative effects. The low and medium doses (20 and 120 μg/ml)
resulted in undetectable blood nicotine concentrations in 4 out of 5
mice. Only mice treated with the highest dose had measurable blood
nicotine levels in most animals (4 out of 5 mice) with an average
concentration of 6.2 ng/ml. Blood nicotine concentrations of up to 21.1
ng/ ml were measured, which are comparable to concentrations seen in
people using nicotine replacement products, smoking a cigarette or using
smokeless tobacco products such as chewing tobacco or snuff [19].
However, variability in the HD groups was high because of the rapid
degradation of nicotine in mice. With increasing nicotine concentrations
in the drinking water, we saw increases in blood cotinine levels, the
major metabolite of nicotine. However, at all three doses, blood
cotinine levels exhibited high variability, which seems to be
characteristic for the oral administration route. At 300μg/ml, blood
cotinine concentrations ranged from 41.4-578.1 ng/ml with an average of
192 ng/ml. These levels are comparable to findings from other groups,
which have shown that in mice treated with nicotine via drinking water
(200 to 500 μg/ml), plasma cotinine levels from 652 to 1450 ng/ml were
found [44,45].
The large variability of plasma cotinine levels between and within
studies is likely due to the relatively short half-life of cotinine in
mice (20-40 minutes) compared to other species (rats 5-6 hours and human
19 hours) [24,25,46,47].
Thus, blood cotinine concentration may not be a reliable surrogate
measure of blood nicotine levels in mice, but does indicate recent
nicotine consumption. Over the years, a number of studies have shown
that chronic nicotine reduces body weight and/or suppresses food intake
in humans and laboratory animals [48-52]. In neonates, even very low doses of nicotine reduces body weight gain [53], and people using nicotine replacement therapy during smoking cessation, also exhibit reduced weight gain [54,55].
In this study, an anorexic effect of nicotine was only observed in the
HD-group, whereas in the LD- group and MD-group, there was no effect on
either food intake or body weight. This is consistent with other studies
which used the oral route of nicotine administration and reported that
chronic treatment with lower nicotine concentrations via drinking water
does not affect weight gain or food intake [56].
Furthermore, nicotine's anorexic effect did not start until treatment
day 8, when the highest dose of nicotine was reached. This result
correlated with more consistently measurable blood nicotine levels in
the HD-group, and suggests that nicotine consumed through the drinking
water can exert central anorexic effects. However, decreases in body
weight and food intake have not consistently been reported with chronic
oral nicotine administration [57], or were only seen after prolonged treatment [45].
It is possible that sufficient blood nicotine levels where not reached
for long enough time periods to trigger an anorexic effect [57], or where only reached after the highest dose was administered [45].
Another hallmark for the central effects of chronic nicotine exposure
is an increase in heteromeric high affinity nicotinic binding sites in
the brain, which have also been observed in oral nicotine administration
models using mice [33,57,58].
Using receptor autoradiography, we determined that only the highest
treatment dose of nicotine increased 125I-epibatidine binding to
heteromeric nAChRs in cortical and hippocampal regions. In contrast, low
and medium doses had no effect, although, cotinine measurements
indicated recent consumption of nicotine. The receptor binding results
correlate well with the lack of consistently detectable blood nicotine
concentrations and lack of an anorexic effect in the LD- and MD-groups,
and suggest that nicotine has to be chronically present at sufficient
levels in order to up regulate nicotinic binding sites. Taken together,
nicotine and cotinine blood levels, food intake, body weight, and
receptor binding results indicate that nicotine consumed via the
drinking solution, is effective in reaching relevant levels of nicotine
in the mouse brain comparable to those seen in human smokers or patients
using nicotine replacement medications. However, due to the rapid
metabolism of nicotine in mice, relatively high levels of nicotine doses
need to be consumed to achieve levels that reliably induce central
effects. Chronic exposure to nicotine can affect anxiety levels in
humans and animals, but the issue is complex. Most human smokers
experience lower anxiety levels than non-smokers when challenged with
stress, and increased anxiety levels during smoking cessation,
suggesting that chronic nicotine exposure can reduce anxiety in humans [34-38].
However, in rodents, nicotine’s effects on anxiety are less consistent.
Chronic or acute nicotine administration can result in either an
anxiolytic or anxiogenic response, depending on several confounding
factors such as age, dose, route of administration, sex and species [37,38].
Nicotine or other nAChR agonists given chronically for medicinal
purpose should not induce anxiety. Very few studies have evaluated the
effects of chronic nicotine exposure administered via drinking water on
behavioral measures. In one such study, female but not male mice
exhibited increased anxiety-like behavior after chronic oral nicotine
administration [59], whereas a sex independent anxiogenic effect was found in wild type mice in another study [60].
Therefore, we evaluated anxiety-like behavior in the HD- group using
the open field test. In this study, locomotor and exploratory activities
were not changed, but two parameters, margin time and thigmotaxic
index, were lower in mice from the HD-group compared to controls,
indicating a trend towards reduced anxiety levels. This indicates that,
chronic oral nicotine treatment with a dose sufficient to up regulate
heteromeric nAChR binding sites and to affect body weight, did not
increase anxiety-like behavior in adult male mice, but might have mild
anxiolytic effects. These findings are consistent with results found in
adult male mice after chronic oral administration of nicotine, which
also did not result in increased anxiety-like behavior [59], but contrast with results from a study which used a lower dose of nicotine [60]. The reasons for the discrepancies between these studies are not clear.
Conclusion
Oral self-administration of nicotine via drinking
water provides a stress-free route of chronic drug treatment, but
requires relatively high doses to reach biological active levels of
nicotine in the mouse brain. Three weeks of nicotine consumption
decreased food intake and body weight, and increased expression of high
affinity nicotine binding sites, hallmarks of central effects of chronic
exposure to nicotine. Furthermore, chronic voluntary oral consumption
of nicotine at levels high enough to exhibit central effects of chronic
nicotine exposure,and did not have anxiogenic effects, making this route
and this dose suitable for long-term treatment of adult mice.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgement
The open access publishing fees for this article have
been covered by the Texas A&M University Online Access to Knowledge
(OAK) Fund, supported by the University Libraries and the Office of the
Vice President for Research.
For more articles in Open Access
Journal of Toxicology (OAJT) please
click on: https://juniperpublishers.com/oajt/archive.php

Comments
Post a Comment