Method Development and Pharmacological study of Chemotherapeutic Agents
Author: Krishnasarmapathy
Abstract
The processes of method deveiopment for the
pharmacoiogicai studies of chemotherapeutic agents (i.e., methoxyamine
method deveiopment by using LC-MS/MS), were illustrated. To be more
specific, a tetra-enzyme cocktail utilized for DNA adducts release was
introduced. LC-MS/MS method for the analysis of methoxyamine modified
DNA a basic sites incorporated in DNA was developed toward the DNA
adducts released from DNA with the enzyme cocktaii. The methods were
appiied to the drug effect and drug mechanism studies. The resuits in
this work not oniy demonstrated the capabiiity of LC-MS/MS in soiving
sophisticated pharmacoiogicai puzzies, but wiii provide usefui
information guiding the preciinicai studies and ciinicai therapy
deveiopment of the anti-cancer drugs. In the deveiopment of anti-cancer
drugs, it is essentiai to study the pharmacological profiles of the
drugs. Among the analytical tools utilized in the pharmacological
studies, LC-MS/MS has gained increased popularity due to its unequivocal
sensitivity and specificity, as well as the ability of handling a wide
variety of compounds with relatively simple sampie preparation
procedures. In this work, a brief review on the method rationai,
instrumentations, anaiyticai method vaiidation, and work flow of the
method development was included. Cancer is a worldwide problem. Finding
novel compositions and methods for treating cancer is of interest. The
treatment of cancer faiis into three generai categories: chemotherapy,
radiation therapy and surgery. Often, therapies are combined since a
combination of therapies increases the probabiiity the cancer wiii be
eradicated as compared to treatment strategies utiiizing a singie
therapy. Typicaiiy, the surgicai excision of iarge tumour masses is
foiiowed by chemotherapy and/or radiation therapy
Pharmacological Studies of Anti-Cancer Drugs
Cancer is a woridwide probiem. Finding novei
compositions and methods for treating cancer is of interest. The
treatment of cancer faiis into three generai categories: chemotherapy,
radiation therapy and surgery. Often, therapies are combined since a
combination of therapies increases the probabiiity the cancer wiii be
eradicated as compared to treatment strategies utiiizing a singie
therapy. Typicaiiy, the surgicai excision of iarge tumour masses is
foiiowed by chemotherapy and/or radiation therapy. Chemotherapeutic
agents can work in a number of ways. For exampie, chemotherapeutics can
work by interfering with ceii cycie progression or by generating DNA
strand breaks [1].
If the cancer ceii is not abie to overcome the ceii cycie biockage or
ceii injury caused by the therapeutic compound, the ceii wiii often die
via apoptotic mechanisms. The use of a singie chemotherapeutic agent in
the treatment of cancer, with or without surgery or radiation, has
severai disadvantages. Commoniy, cancer ceiis deveiop resistance to the
chemotherapeutic agent. Such resistance resuits either in the
requirement for higher dosages of the drug and/or the renewed spread of
the cancer. Chemotherapeutic agents can be toxic to the patient.
Therefore, there is a practicai upper iimit to the amount that a patient
can receive. However, if a second agent can be deveioped to inhibit the
pathway causing resistance, cancer ceiis may become susceptibie to the
effects of the chemotherapeutic agent [2].
The design of a drug to overcome resistance to the chemotherapeutic
treatment of cancer shouid be approached with the goais of:
a. Finding a combination that reverses resistance and
not mereiy improves the activity of the chemotherapeutic with respect
to activity on the tumour, and
b. Finding a second drug that does not potentiate the toxic effects of the first chemotherapeutic agent
LCMS-MS
Detection and quantification-HPLC/MS/MS analysis
UDG-/- and UDG+/+ cells were exposed to pemetrexed
(10µM) or 5-fluorouracil (10µM) for 6, 24, 48 and 72hrs. At indicated
time points, ceiis were harvested and genomic DNA was extracted by
phenol/chloroform. 40µg of DNA were incubated with 10 U of purified UDG
(New England Biolabs) in 60µL of reaction buffer at 37 °C for 2hrs. The
reaction products were dried at 35 °C in a Turbovap under a stream of
nitrogen and reconstituted in 150µL 90% acetonitrile. The analyse was
retained by an Atiantis Hiiis Siiica anaiyticai coiumn (2.1x100mm,
3.5µM) and eluted isocratically by a mixture of 90% acetonitrile and 10%
2.0mM ammonium format at a flow rate of 0.2ml/min. The detection was
done by an API 3200MS/ MS mass spectrometer.
Material and Methods
Chemicals and solutions
MX•HCl, 2-deoxyribose, ribose, ammonium format,
formic acid, isopropanoi, acetonitriie, BisTris, CT-DNA, O-(4-
Nitrobenzyl) hydroxylamine, ethanol, sodium citrate, DNase I from bovine
pancreas, NP1 from Peniciiiium citrinum, bovine intestinai ALP, and
Tris EDTA buffer were obtained from Sigma- Aldrich (St. Louis, MO).
Triethylamine, NaH2PO4, NaCl, ZnCl2, PBS, water saturated phenoi, and chioroform were purchased from Fisher Scientific (Fair Lawn, NJ) [3].
Tris base and 10% SDS were from Bio-Rad Laboratories (Hercules, CA).
DMEM medium and L-glutamine were from Media tech (Manassas, VA). RNase
and protease K were from Invitrogen (Carlsbad, CA). DNA 11- mers
5’-GCCGT-U-AGGTA-3’ and 5'-AGGTAGCCGT-U-3' were synthesized by
Integrated DNA Technologies (Coralville, IA). Methanol was purchased
from Pharmco- AAPER (Brookfield, CT). 1,1,1,3,3,3-Hexafluoro-2-propanol
was obtained from Oakwood Products (West Columbia, SC) [4]. Uracil DNA glycosylase (with 10x enzyme buffer) was from New England Biolabs (Ipswich, MA) [5]. Acetic acid was from Mallinckrodt Baker (Phillipsburg, NJ). Hydrochloric acid was from EMD Chemicals (Gibbstown, NJ) [6]. Snake venom PDE I was obtained from Worthington Biochemical Corporation (Lakewood, NJ) [7]. Fetal bovine serum was purchased from Hyclone Laboratories (Logan, UT) [8]. TMZ was from Ochem (Des Plaines, IL) [9]. Deionised water was prepared by the Barnstead NANO pure® water purification system (Thermo Scientific, Waltham, MA, USA) [10].
Synthesis of MX-dR and MX-R
MX•HCl powder was dissoived in deionised water to a
concentration of 1.0M. 2'-Deoxyribose and ribose powders were dissoived
in deionised to make 1.0M soiutions, respectiveiy. Then each
concentrated soiution was diiuted with deionised water to a
concentration of 10mM [11].
To synthesize MX-dR, 10µL of MX•HCl solution (1.0M), 10µL of deoxy
ribose solution (10mM) and 80µL of deionized water were pipette together
into a 0.5mL micro centrifuge tube. Then the tube was kept in 70 for
2h. For the synthesis of MX-R, the IS, the 1 M MX•HCi soiution was
diiuted with de-ionized water to 10mM. Then, 10µL of MX•HCl solution
(10mM), 10µL of ribose solution (1.0M) and 80µL of de-ionized water were
pipette together into a 0.5mL micro centrifuge tube [12].
Then the tube was kept in 70 again for 2h. Both reactions were stopped
by 100xdiiution with deionised water. Then, the reaction products were
kept in -4 tiii use.
LC-MS/MS and LC-MS instrumentations
The instrument system inciuded a Shimadzu HPLC system
(Kyoto, Japan) composed of a solvent reservoir, a degasser (DGU- 20A3),
a binary pump (LC-20AD) [13],
a flow controller (CBM- 20A), and an auto sampler (SIL-20ACHT),
together with an AB SCIEX 5500 QTRAP® 5500 mass spectrometer (Foster
City, CA) controlled by Analyst software (version 1.5.1) [11].
LC-MS/MS of MX-dR and the IS
Chromatographic separation was carried out on a
Thermo (West Palm Beach, FL) Hypercarb TM column (2.1x50mm, 5|iM) at
ambient temperature (23) with a flow rate of 0.4mL/min. A two-soivent
gradient, 5mM ammonium format [14]
(NH4Fc, pH 3.5) (A)and 5mM NH4Fc (pH 3.5) in 67% methanol and 33%
isopropanol (v/v) (B), was utilized for complete separation of MX-dR
from the matrix interferences. At the beginning of the LC, 100% A was
held for 1.0min. Then the content of A was dropped quickly from 100% to
40% within 1.0 to 1.1min. Next, the mobile phase was held at 40% A till
5.9min, followed by returning to 100% A at 6.0min. Before each run,
there was an equilibration set as 5min. The coiumn eiuent was diverted
to the waste before 2.49min, and then to the mass spectrometer between
2.50min and 3.10 min. At 3.11 min the flow was diverted to the waste
again tiii the end of the run [15].
The mass spectrometer was operated at the positive-eiectro spray-ionization (ESI+) mode [16].
It was tuned by flow injection of a mixture of MX-dR (100ng/mL) and
MX-R (100ng/mL) in the mobile phase (60% B) at a flow rate of 0.4mL/min.
The source dependent parameters were as follows: curtain gas TM (CUR),
20; ion spray voltage (IS), 5500; temperature (TEM), 300; gas1 (G1),
60.0; gas 2 (G2), 60.0 [17].
The compound dependent parameters were as follows: De-clustering
Potential (DP), 50.0; Entrance Potential (EP), 8.00. Detection of MX-dR
and MX-R was based on MRM with the conditions set as foiiows: Coiiision
Gas (CAD), low; Collision Energy (CE), 10.0; Collision Cell Exit
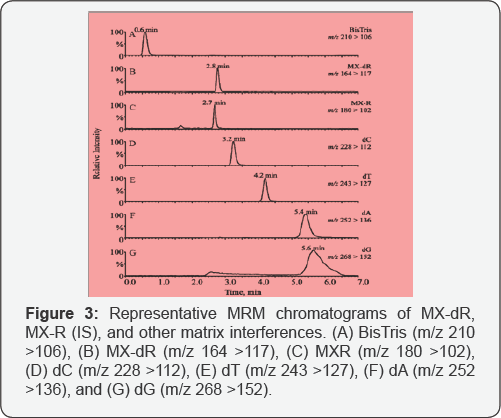
Potential (CXP), 11.0; Dewell Time, 100ms [18]. Two MRM channeis: m/z 164 > 117, and m/z 180 > 102 were utiiized to monitor MX-dR and MX-R, respectiveiy.
LC-MS of the oligo nucleotides
The chromatography separation of the oiigonucieotides
was carried out on an Xterra MSC18® column (2.0x50mm, 3.5|im, Waters,
Milford, MA) by adjusting an existing method [19].
Isocratic separation was performed at ambient temperature (23) at a
flow rate of 0.2mL/min with a mobile phase containing 86% 200mM
1,1,1,3,3,3- Hexafluoro-2-propanol [HFIP, adjusted to pH 7.0 with
N,N,N-Tri ethylamine (TEA)] and 14% methanol (v/v). The column eluent
was diverted to the waste before 2.00 min, and then to the mass
spectrometer at 2.00min.
Negative-electro spray-ionization (ESI-) mode MS was
operated with the source dependent parameters as the foiiowing: CUR, 20;
IS, -4500; TEM, 400; G1, 40; G2, 40.The compound- dependent parameters
were as foiiows: DP, -100; EP, -10. DNA 11-mers 5’-GCCGT-U-AGGTA-3’ and
5'-AGGTAGCCGT-U-3', 5'-GCCGT-AP-AGGTA-3', as well as
5’-GCCGT-(MX-AP)-AGGTA-3' were monitored with the Q1 Ml scan mode
(selected reaction monitoring or SIR) in channel m/z 670.5 (M-5H), m/z
651.8 (M- 5H), and m/z 657.5 (M-5H), respectively. The Dewell Time was
set as 100 ms for each channel. The DNA 11-mers were diluted with
de-ionized water to 1µg/mL. For each analysis, 2µL of sample was
injected onto the column.
Results and Discussion
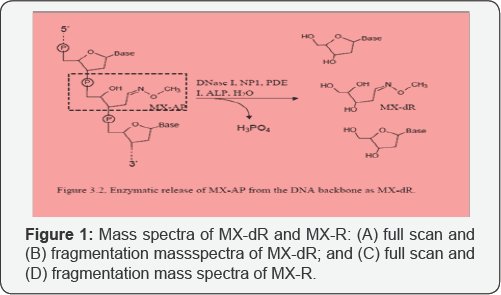
Characterization of MX-dR and the IS with mass
spectrometry Since MX-AP is bound to DNA strand, to realize the
quantification, a tetra enzyme system was utilized to release MX-AP as
MX-dR (Figure 1 & 2).
By carrying out this enzymatic digestion, the quantification of MX-AP
bound to DNA was converted to the quantification of the free small
molecule, MX-dR. To achieve highly accurate and repeatable results,
another small molecule, MX-R was synthesized as the IS [20].
After reaction, the two post-reaction mixtures were diluted with 5mM
NH4Fc for 100 times, respectively, and infused into the mass
spectrometer by a syringe pump at a flow rate of 5µL/min. As both MX-dR
and the IS are more easily to form protonated species through ESI, ESI+
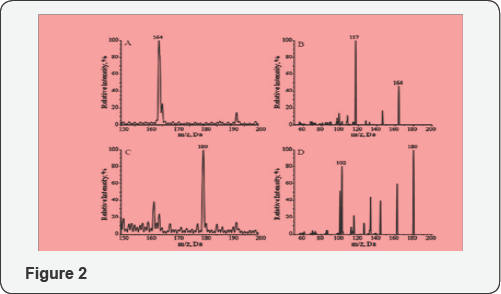
mode was utilized. As shown in Figure 3,
MX-dR and the IS produced molecular ions at m/z 164 ((MX-dR+H)+) and
m/z 180 ((MX-R+H)+), respectively. To achieve higher specificity in the
quantification, the molecular ions were further dissociated with CID.
From the resulted fragmentation pattern, two predominant fragments were
observed at m/z 117 for MX-dR and m/z 102 for the IS, respectively.
Therefore, the mass transition pairs m/z 164>117 for MX-dR and m/z
180>102 for the IS were utilized in the quantification work with MRM
mode. Figure 2, Enzymatic release of MX-AP from the DNA backbone as MX-dR.
Digested DNA sample extraction
As the quantification was carried out toward the
MX-dR in the enzyme digested DNA samples, several major interferences
were expected: buffer salts (i.e., 4.69mMM BisTris, 145µM NaCl, and
14.1µM ZnCl2), proteins (i.e., protein impurities existing in the DNA
samples and enzymes utilized in the digestion), and the dNs (with a
total concentration of around 3mM). To avoid signal suppression and ion
source contamination caused by these interferences, the analyse must be
effectively separated from these interferences through on-line and/or
off-line procedures. Two off-line extraction methods were tried in order
to remove the matrix interferences.
A LLE method with a mixture of ethyl acetate and
isopropanol (95:5, v/v) was tried. This method was effective in removing
NaCl and ZnCl2. It was also able to remove over 90% BisTris. However,
it failed to eliminate the dNs effectively. An SPE method with a caution
exchange cartridge, the Oasis® MCX cartridge, was utilized under the
intention of retaining all the dNs on the cartridge, yet collecting
MX-dR from the cartridge pass-trough. To retain dA, dC, and dG, moderate
acidic pH (i.e., pH 4) had to be utilized in sample loading. Under the
same pH, however, dT was predominantly negatively charged, and could not
be retained on the cartridge [21].
Besides, extra steps were still needed to separate the analyse from the
buffer salts. As a result, the samples were simply processed by
one-step acetonitrile precipitation to remove the proteins. Removal of
the buffer salts and the dNs was left as a task in the LC method
development.
LC separation of the analyse from the matrix interferences
Several columns (i.e., an YMC ODS-AQ® column, an
Xterra MSC18® column, and a HypercarbTM column) were tried to obtain the
separation between MX-dR and the other interference compounds. The YMC
ODS-AQ® column was tried due to its capability of retaining highly polar
compounds, and its compatibility with highly aqueous mobile phases.
However, even when the percentage of the organic component (i.e.,
methanol) was dropped below 2%, no significant retention was observed
for MX-dR. As MX-AP adduct can also be converted to MX-deoxy ribose
5'-phosphate (MX-dRp) in the enzymatic releasing of MX-AP through
laminating ALP from the enzyme cocktail, ion-pairing chromatography with
TEA was considered. In this test, the Xterra MSC18® column was
utilized. By adjust the pH and the organic percentage of the mobile
phase, MX- dRp could be retained on the column for up to 3 column
volumes without causing significant tailing. However, all the
2'-deoxyribonucleotide mono phosphates (dNMPs) released after enzyme
cutting could not be separated from the analyse effectively. In the work
of Antonio et al. several sugar and sugar phosphates were separated on a
porous graphitic carbon (PGC) column, the HypercarbTM column [22].
Because the graphite surface possesses a large amount of delocalized
n-electrons, it is easy to induce electronic interaction with the
analytes carrying polarisable or polarized groups [23].
And thus, the columns can provide strong retention to highly polar
compounds. Another advantage of the PGC columns lies in their pH
stability: they are stable throughout the pH rang as the structure of
MX-dR is similar to those of the sugars, separation between the analyse
and the matrix interferences was tried on this column. Isocratic elusion
with a mixture of NH4Fc and organic solvents (i.e., methanol,
acetonitrile, isopropanol, methanol/acetonitrile, or
methanol/isopropanol) was able to retain MX-dR for at least 2 column
volumes and achieve single symmetrical peak at the same time. Some of
the conditions were also able to separate the analyse effectively from
all the dNs and the inorganic salts. However, the separation between
BisTris and MX-dR was always not enough due to the column's slightly
retention to BisTris. Better separation between MXdR and BisTris can be
achieved through utilizing lower percentage of weaker organic solvents,
such as methanol, but the peak of MX-dR started to split. Besides, the
retention times of the dNs were increased significantly (over 60min for
dA and dG). Based on these reasons, a gradient elusion with the
conditions described was finally adopted. With this LC method, MX-dR was
able to be retained on the column for 2.8min, while all the dNs were
eluted out after 3.2min (Figure 4). The BisTris was eluted out at 0.6 min. By applying the same LC condition, the IS was eluted out at 2.7min.
MX-AP DNA standard preparation
Since the quantification of MX-AP was realized
through quantification of MX-dR released after enzyme digestion, DNA
spiked with synthesized MX-dR would not be reliable in accurate
quantification due to its invalidity in reflecting the digestion
efficiency. Neither would the MX-AP DNA synthesized according to the
protocol be reliable standards due to the uncertain amount of MX-AP
sites it carries from batch to batch. Based on these considerations, a
single strand DNA 11-mer with one MX- AP adduct located in the middle
(i.e., 5'-GCCGT-(MX-AP)-AGGTA, the MX-oligo) was synthesized by
modifying an existing protocol for AP-oligo synthesis [24].
Spiking the MX-oligo into blank CT- DNA resulted MX-AP DNA calibrators
carrying all the necessary information required by the accurate
quantification.
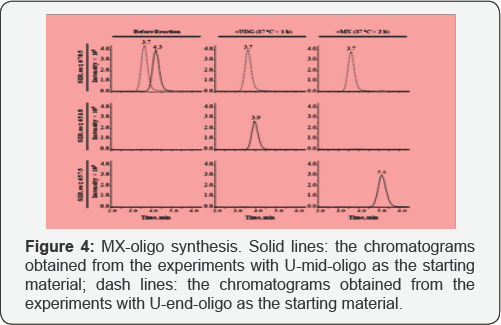
To monitor the synthesis process of MX-oligo, the
starting material and product of each step of reaction were analyzed
with the LC-MS method (Figure 5).
As the remaining reactant was less than 1% of the original amount after
each step of reaction, both steps were considered as complete. The
U-mid- oligo was converted to equal amount of MX-oligo. To avoid high
quantification background caused by the reaction between the excessive
MX in the synthesis product and the AP sites generated through
spontaneous hydrolysis during the enzyme digestion, the MX-oligo was
purified on an Oasis® HLB SPE cartridge with a protocol adjusted from a
published method [20].
By comparing the peak area of MX-oligo from the reaction product before
and after purification, the recovery was determined as 94.2±1.6%.
As UDG is unable to remove uracil from the end of a
DNA strand, UDG digestion on the U-end-oligo is not effective in
producing AP-oligo (Figure 5).
Further reaction with MX was not able to generate MX-oligo efficiently
as well. Thus, the U-end- oligo was processed parallel with the
U-mid-oligo as a negative control of the studies. In another word, the
U-mid-oligo is able to reflect any background caused by excessive MX
remaining in the purified oligomer products, or the background caused by
MX-AP adducts formed from the reaction between MX and the AP sites
generated spontaneously during the oligomer synthesis.
Method performance
To evaluate the performance of the developed methods
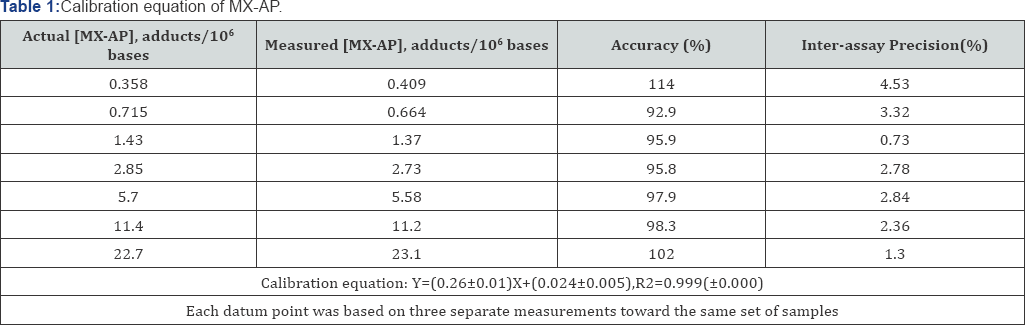
in quantitative studies, a calibration curve was established with a
linear calibration range of 0.358-22.7 MX-AP adducts/106 bases. The
curve was weighted by the reciprocal of MX-AP concentration,1/x. The
calibration equation has been shown in Table 1, and the linearity, represented by correlation coefficient R2,
was 0.999±0.000. The accuracy and inter-assay precision of each point
on the calibration curve ranged from 93.6-115% and 0.73-4.53%,
respectively (Table 1).
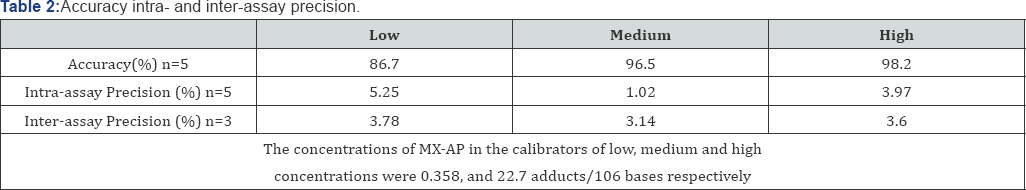
The accuracy, intra-assay precision, and inter-assay
precision of the analysis were determined through the quintuplicate
calibrators at three concentration levels (low, medium, and high). All
data were summarized in Table 2.
The accuracy ranged from 86.7-98.2%; while the intra-and inter-assay
precision varied from 1.02-5.25% and 3.14-3.78%, respectively. Here the
accuracy was calculated by the relative deviation between a calculated
concentration and the nominal concentration; while the precision was
calculated by percent standard deviation.
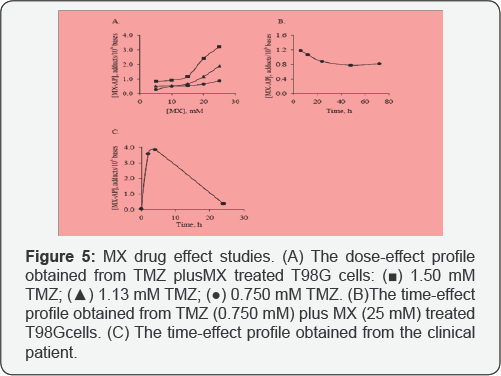
Analysis of TMZ plus MX treated T98G cells in the cellular DNA analysis
In this section, the MX-AP concentration of every
real sample was normalized with the method. The reason of carrying out
this normalization procedure lies in its advantages in more accurate
quantification. T98G cells treated with TMZ plus MX with different
dosages and time spans were analyzed with the developed methods. A
dose-effect profile and a time-effect profile were obtained afterward.
From the dose-effect profile, a clear relationship between the dosage of
TMZ plus MX and the concentration of MX-AP can be observed. For each
dosage level of TMZ, the concentration of MX-AP was elevated with the
increase of the MX dosage. Meanwhile, when the dosage of TMZ was
increased, the profile of response was lifted systematically. These
results are consistent with our hypothesis: higher concentration of MX
blocks more AP sites; while higher concentration of TMZ generates more
AP sites systematically. From the time-effect profile, when the
treatment time increased from 6h to longer, the concentration of MX-AP
decreased slightly at the beginning, and then reaches a relatively
steady state after 24h treatment.
Analysis DNA samples from TMZ plus MX treated patient
The DNA samples from the patient with solid tumour
enrolling in the phase I clinical trial of TMZ plus MX drug combination
were analyzed. The time-response profile has been illustrated in.
Determined from the profile, the concentration of MX-AP quickly reaches
to the maximum after 4h treatment, and then decreased gradually below
0.500 MX-AP adducts/106 bases after 24h. The quick response
of the patient to the treatment was consistent with our in vitro result.
The clearance rate of MX-AP adducts, however, was much higher in the
patient comparing to the cultured cells.
The reason for the difference is still under
investigation. In fact, DNA samples from 4 patient enrolled in the phase
I clinical studies were analyzed with our method, and only this one
showed detectable signals for MX-AP. The PK profile of the patient
indicated a significantly lower blood concentration of free MX. This may
indicate extremely high MX incorporation into the patient's DNA.
However, during the drug effect analysis, the amounts of MX-AP detected
from the real samples were much lower comparing to the expectation.
Under physiological conditions, DNA bases can be dissociated from the
DNA strand through spontaneous hydrolysis at rate of 2 bases/106
bases in every 24h. Under the treatment of TMZ, although the amount of
AP site generated after treatment has not been evaluated, it should be
much higher than the AP sites generated by spontaneous hydrolysis.
Assume that addition of MX will lead to 1 MX-AP adduct/106
bases after 24h treatment; at the same time, the white blood cell count
in of the patient is 5x106 cells/ mL blood. As the blood drawn from each
time point was around 5mL and the extracted DNA samples were typically
dissolved in 15|iL of BisTris buffer before digestion, a calculation on
the total amount of MX-AP adduct in the patient DNA samples can be
roughly estimated as the following:
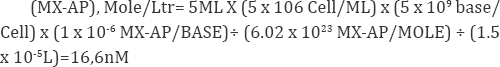
Since the total concentration of the dNs in a
digested DNA sample is around 3.2mM, if convert the detection limit of
MX-AP from standards to mole concentration:
(MX-AP), Mole/Ltr= (0.358 x 10-6 MX-AP/BASE) x 3.2Mm=1.15nM.
Based on the calculation, the MX-AP signal should be
detectable in the patient DNA. However, due to the patients enrolled in
the clinical trial were with solid tumour, the white blood cell count in
these patient may be much lower than healthy donors. Meanwhile, as the
cellular DNA was extracted in a relatively inefficient way, the recovery
of the DNA may well below 100%. Last but not the least, the DNA
extracted from the real samples can be easily contaminated with histon,
SDS, and protease K [18].
All three kinds of impurities may greatly inhibit the releasing
efficiency of the enzyme cocktail on the MX-AP adducts. In another word,
before applying the developed method into the analysis of patient DNA
samples, further optimizations must be carried out. The optimization can
be done in two aspects. First, the DNA extraction and purification
method can be further optimized. DNA extraction kit can be utilized for
higher recovery and purity, as well as more reproducible results from
sample to sample. Second, the enzyme digestion conditions can be further
optimized. If necessary, different enzymatic system can be
experimented.
Acknowledgement
The authors are thankful to TORRENT-Heuman pharma
GMBH, CDRI Lucknow Pharmaceutical Industries Ltd, India for providing
the standard and providing the research facilities
Conclusion
A tetra-enzyme cocktail containing DNA phase I, NP1,
PDE I and ALP has been utilized for the quantitative releasing of MX- AP
from the DNA backbone as MX-dR. A protein precipitation procedure was
utilized in the sample preparation to remove protein interferences in
the sample matrix. LC separation was realized on a HypercarbTM column
for further separation of MX-dR and the IS from all the other
interferences existing in the sample matrix. The MX-AP calibrators were
prepared by spiking DNA 11-mers with one MX-AP site on each 11-mer into
blank CT-DNA. A calibration curve ranged from 0.358-22.7 MX-AP
adducts/106 bases were established, and the accuracy and precision were
evaluated through a set of quality control calibrators. The feasibility
of the method was tested by drug effect studies both in vitro (i.e.,
with the TMZ plus MX treated T98G cells)and in vivo (i.e., with
the lymphocytes of the clinical patient treated with TMZ plus MX in a
phase I clinical trial). The method we developed provided direct
information on drug effect of MX, and thus will assist in providing
dosimetric guidance in future clinical trials.
For more
details Open Access Journal of Toxicology (OAJT) please
click on: https://juniperpublishers.com/oajt/index.php
To read more…Full Text
in in Juniper Publishers click on https://juniperpublishers.com/oajt/OAJT.MS.ID.555568.php

Comments
Post a Comment