Rat Brain Acetyl Cholinesterase as a Biomarker of Cadmium Induced Neurotoxicity
Abstract
Cadmium as potential environmental xenobiotics has
been shown to cross blood brain barrier and to adversely influence the
activity of AChE and hence the brain functions. In the present study, we
have evaluated the impact of cadmium in vitro on the properties
of AChE isolated from rat brain. The enzyme was found to be membrane
bound and it could be successfully solubilized using 0.2% (v/v) Triton
X-100, a nonionic detergent, in the extraction buffer (50mM Phosphate,
pH 7.4). The enzyme was found to be highly stable up to one month when
stored at -20°C. This enzyme exhibited maximum activity at pH 7.4. AChE
when incubated at different temperatures for 5 min, displayed maximum
activity at 37°C. Treatment with higher temperatures caused inactivation
of enzyme activity. The enzyme followed a simple Michaelis-Menten curve
when assayed at varying substrate concentration and yielded Km
value to be 0.0370 mM. When a fixed activity of AChE was assayed in
presence of different concentrations of cadmium, the enzyme activity was
sharply reduced; the IC50 value being about 5.7mM. The enzyme when assayed in presence of cadmium at a concentration equal to its IC50, lost its 50% activity in 77 min (t1/2
). Cadmium was found to act as a noncompetitive inhibitor to the
enzyme. These results suggested that AChE from rat brain may serve as a
significant biomarker of cadmium induced neurotoxicity.
Keywords: Acetyl cholinesterase; Brain; Cadmium; Biochemical properties; Inhibition
Abbreviations:
RoHS: Restriction on Hazardous Substances; Ach: Acetylcholine; Cd:
Cadmium; ATI: Acetylthiocholine Iodide; DTNB: 5, 5'-dithio-bis (2- nitro
benzoic acid).
Introduction
Cadmium a most abundant heavy/transition metal, discovered by Fridrich Strohmeyer [1]
in 1817 as an impurity in zinc carbonate or colamine. According to the
Dmitri Mendeleev's modern periodic table, it falls under group IIB,
period 5, having atomic number 48. It is soft bluish white d-block
element, chemically similar to the zinc and mercury. This metal does not
have any known useful function in the human body and produces harmful
effects once it enters the body through inhalation, ingestion and skin
contact. Cd can replace iron and copper from a number of cytoplasm and
membrane proteins like ferritin, thereby causing rise in the iron and
copper ions concentration, which may be associated with the production
of oxidative stress via Fenton reaction [2,3].
Cd toxicity may be carried out by the proteins having zinc finger
motifs into its structures. Due to the similarity between zinc and
cadmium, cadmium can easily replace zinc in biological systems
(particularly systems which have -SH containing ligands) and binds ten
times more strongly than zinc in biological systems therefore it is
difficult to remove. On the other hand, it is also reported that cadmium
may also replace magnesium and calcium ions in biological systems,
though such replacements are very rare [4,5].
Cadmium is an important component of making batteries, cadmium pigments
and coatings and plating and as stabilizers for plastics, chemical
stabilizers, metal coatings, alloys, barrier to control neutrons in
nuclear fusion, black and white television phosphors, and blue and green
phosphors for color television picture tubes, and semiconductors and in
molecular biology to block voltage- dependent calcium channels from
fluxing calcium ions. Cadmium poisoning is an occupational health hazard
associated with industrial processes such as metal plating and the
production of nickel-cadmium batteries, pigments, plastics, and other
synthetics. Adverse effects of human exposure to cadmium were first
established among workers in a cadmium battery factory [6].
Workers are exposed occupationally to cadmium primarily by inhalation
of fumes or dust. Some gastrointestinal tract exposure may also occur
when dust is removed from the lungs by muco ciliary clearance and
subsequently swallowed, or by ingestion of dust on hands, cigarettes, or
food [7].
The main sources of exposure to cadmium are specific professional
atmospheres, diet, drinking water, and tobacco. The primary route of
exposure for the general population is through the diet. Also, many
other toxic compounds in cigarette smoke make it difficult to attribute
specific adverse effects of smoking to the inhalation of cadmium fumes
which can result initially in metal fume fever but may progress to
chemical pneumonitis, pulmonary edema, and death. In general, the
different forms of cadmium have similar toxicological effects by the
inhalation route, although quantitative differences may exist from
different absorption and distribution characteristics, particularly for
the less soluble cadmium pigments such as cadmium sulfide and cadmium
selenium sulfide [8].
Because of its carcinogenic property (classified Number one category of
carcinogen by The International Agency for Research on Cancer of USA)
cadmium has been banned by the European Union's Restriction on Hazardous
Substances (RoHS) which causes cancers of lung, prostrate, pancreas,
and kidney. It can also cause osteoporosis, anemia, non hypertrophic
emphysema, irreversible renal tubular injury, eosinophilia, anosmia, and
chronic rhinitis. The generation of ROS by Cd has been one of the known
mechanisms by which this heavy metal induces mutagenesis [9].
Acetyl cholinesterase (AChE, EC 3.1.1.7) or acetyl hydrolase is a
serine cholinesterase that hydrolyzes the neurotransmitter acetylcholine
to be acetyl Co A. and choline. AChE is found mainly at neuromuscular
junctions and cholinergic brain synapses, where its activity serves to
terminate synaptic transmission and is synthesized in the endoplasmic
reticulum and is then exported towards the cellular surface, where its
different molecular/globular forms may be anchored in plasma membrane,
attached to the basal lamina (asymmetric collagen-tailed forms) or
secreted as soluble molecules (non-globular) forms [10].
It is a key enzyme of nerve impulse transmission and is reported to be
inhibited by Cadmium. AChE is an enzyme which occurs at high specific
activity in the brain and in nervous tissues and it is readily detected
in the membranes of muscles and erythrocytes. The most widely adopted
solubilization methods for mammalian brain AChE have involved the
application of detergents, particularly Triton X-100, a non-ionic
detergent [11]. AChE has been widely exploited as a primary target of action by organophosphorus compounds such as nerve agents [12].
AChE has been the focus of much attention since it was first suggested
that it plays an important role in the rapid destruction of
acetylcholine (ACh) in a living organ [13].
The catalytic properties, and their occurrence, histochemical
localization, and molecular heterogeneity in the different tissues of
different animal species have been extensively studied [14-18].
Since cadmium has been found to cross blood brain barrier in mammals
and influence the brain functions, it was imperative to evaluate in vitro
the impact of cadmium on the biochemical behavior of AChE in order to
understand its mechanism of action. In the present study, we have
endeavored to characterize AChE from the rat brain and to monitor its
interactions cadmium under different experimental conditions. The
results have indicated that cadmium may adversely influence brain
functions through modulation of AChE activity. Thus, rat brain AChE may
be exploited as a key biomarker to assess cadmium toxicity
Materials and Methods
Chemicals
S-acetylthiocholine iodide (ATI) and the coloring
reagent 5, 5’-dithio-bis (2- nitro benzoic acid) (DTNB) were procured
from Tokyo Chemical Industry Co., Ltd. Tokyo, Japan and SRL Pvt. Ltd.
Mumbai, India, respectively. Triton X-100 was purchased from Merck.
Bovine serum albumin, Phosphate buffer salts (Sodium dihydrogen
orthophosphate and di-Sodium hydrogen phosphate) were obtained from
Fisher Scientific and Folin and Ciocalteu's Phenol reagent from
Spectrochem Pvt. Ltd. Mumbai, India. All other chemicals were of
analytical grade purity.
Animals
Male albino rats of same age group, weighing between
180210 g were selected for all these experiments. Animals obtained from
CDRI, Lucknow, India, were housed in propylene cages at temperatures of
30±5°C and 45±5% relative humidity with 12h of light and dark cycle.
Animals were fed with standard rat feed available commercially with free
access to water. Protocols for care and maintenance of the rats were
strictly followed and the study had the approval of institutional
ethical committee.
Collection of brain tissues and preparation of homogenates
The healthy rats were sacrificed using mild
chloroform anesthesia and cervical dislocation causing minimal pain. The
whole brain was quickly excised, washed with isotonic ice cold 0.9%
(w/v) NaCl solution, blotted to dryness and weighed. Rat brain tissue
homogenate (10% w/v) were made in 50 mM Sodium Phosphate buffer (pH 7.4)
containing 0.2% (v/v) Triton X-100 and another without detergent using
Potter- Elvehjam homogenizer fitted with a Teflon coated pestle under
ice cold condition (4°C). The homogenates were centrifuged at 9000xg for
30 min using REMI refrigerated centrifuge. The supernatants were
removed and the pellets were reconstituted in equal volume of
homogenizing buffer. Both the supernatants and the pellet's suspensions
were used for protein estimation and determination of AChE activity. For
determination of IC50, effect of substrate, temperature, pH,
and mode of inhibition the supernatant of rat brain homogenate
containing Triton X-100 was used.
Protein estimation
The protein was estimated using Folin and Ciocalteu's Phenol reagent [19]. The bovine serum albumin was used as a standard. The absorbance of blue colored complex was monitored at 620nm.
Acetylcholinesterase Assay
The activity of AChE in the brain was determined by method described by Ellman et al. [20].
The reaction mixture (3 ml) in quartz cuvette having 1cm path length
contained 0.50mM of ATI, 0.5 mM of DTNB and 50 mM phosphate buffer (pH
7.4). The change in optical density was measured at 412 nm for 3 min at
each interval of 30 sec. The AChE activity was calculated using
extinction coefficient 13.6x103M-1 cm-1 and expressed as mmoles of acetylthiocholine (ATI) hydrolysed ml-1 min-1 or units (U). The specific activity of enzyme was expressed in U mg-1.
The enzyme assays were performed on UV-Visible double beam
spectrophotometer (Thermoscientific Spectroscan UV 2700). The catalytic
activity is measured by the increase of the yellow anion,
5-thio-2-nitrobenzoate, produced due to reaction of thiocholine with 5,
5’-dithio-bis-(2-nitrobenzoic acid) (DTNB). The assay system without
substrate or enzyme was considered as a substrate or enzyme blank,
respectively, and any change in absorbance min-1 recorded in this
condition were subtracted from the experimental observations.
Effect of substrate concentration on AChE activity
The kinetic parameters Michaelis- Menten constant (km) and maximum velocity (Vmax),
were estimated by assaying the enzyme activity using varying substrates
concentrations (acetylthiocholine iodide, ATI) (from 0.00 to 2.00 mM)
and constant enzyme concentration (132|ig) at room temperature (26±2
°C).
Determination of effect of time on cadmium mediated inhibition of AChE
The enzyme (132|ig) was assayed in the presence of 0.
25mM cadmium at various time periods (0 to 120 min) at room temperature
(26±20C) and the residual enzyme activity was monitored. The
activity of enzyme was also recorded at these time points in absence of
Cadmium, which served as a control. The reaction rate measured soon
after mixing the enzyme with other reagents without any further
incubation was used as zero time reaction. The data of percent residual
activity and the time of incubation in min were extrapolated at Y and
X-axes, respectively. The t1/2 value (the time at which the enzyme activity remains half of the original under this condition) was calculated from this plot.
Estimation of IC50 value for cadmium
The enzyme (132μg) was assayed in the presence of
different concentrations of cadmium nitrate and the residual activity
was monitored. The activity recorded in absence of cadmium was
considered as 100%. The IC50 value was calculated by
extrapolating the data taking percent residual activity on Y-axis and
the varying cadmium concentrations at X-axis on a graph.
Determination of mode of inhibition of AChE by cadmium
The enzyme (132 μg) was assayed at varying
concentrations of ATI at room temperature (26±2°C) in the absence and
presence of cadmium (1.0 mM). The Ki and Vmax values were
calculated using the intersections by the straight line at Y and at the
negative abscissa of X-axes, respectively, of the Lineweaver- Burk's
double reciprocal plot. 2.10.
Determination of K value for cadmium in mode of inhibition of AChE from rat brain
The mode of inhibition of enzyme by cadmium was determined by assaying the enzyme mentioned as above using the formula of either
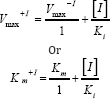
where V max+I and V max-I the maximal velocities of reactions in
the presence and absence of cadmium, respectively. Similarly, Km +I and K m
denote the Km values in the presence and absence
mm
of cadmium, respectively. [I] represents the concentration of inhibitor
used i.e. 1.0 mM. The Ki value may also be calculated using
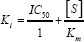
Statistical analysis of data
Statistical analysis of data was performed using
Graph Pad Prism version 6 for windows. All values were expressed as mean
standard deviation of 3 observations.
Results
Membrane bound nature of AChE from rat brain tissue
The enzyme protein content of brain homogenate from
the rat brain tissue were solubilized using a nonionic detergent, 0.2%
(v/v) Triton X-100 in phosphate buffer (50mM, pH 7.4). The extent of
enzyme activity was more in the detergent solubilized fraction than that
of without detergent. These results demonstrated the membrane bound
nature of this enzyme. The protein contents in the soluble fractions of
these two preparations were also found to be significantly different.
The fraction obtained supernatant with Triton X-100 contained 3.30 mg/ml
protein as against 1.20 mg/ml in the fraction without treatment with
the detergent. The pellet with Triton X-100 contained 1.15 mg/ml protein
against 3.15 mg/ml in the fraction without treatment with the
detergent, thereby showing solubilization and release of proteins from
the pellets in presence of the detergent (Table 1).
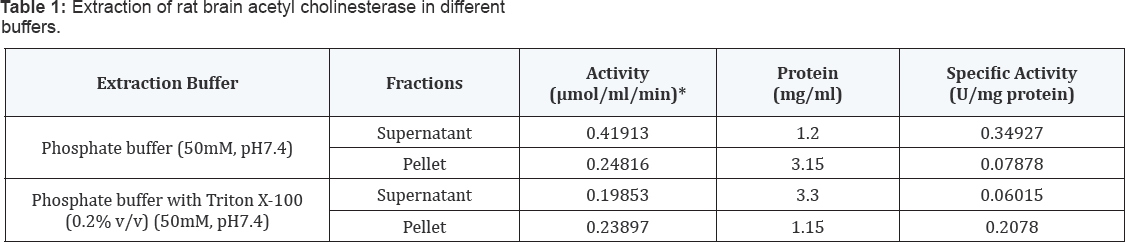
*One unit of the activity of AChE has been defined as the micromoles of substrate hydrolyzed per min per ml.
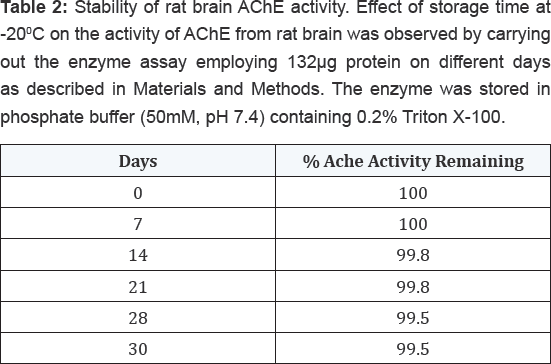
Stability of enzyme activity after storage at -200C
The effect of storage time at -200C on the
activity of AChE from rat brain tissue was determined by carrying out
the enzyme assay employing 132μg proteins on different days as described
in Materials and Methods. The enzyme was stored in phosphate buffer
(50mM, pH 7.4) containing 0.2% (v/v) Triton X-100. The results shown in Table 2
indicated that the enzyme was highly stable up to 30 days with no much
loss in activity. However, when this enzyme was assayed at varying
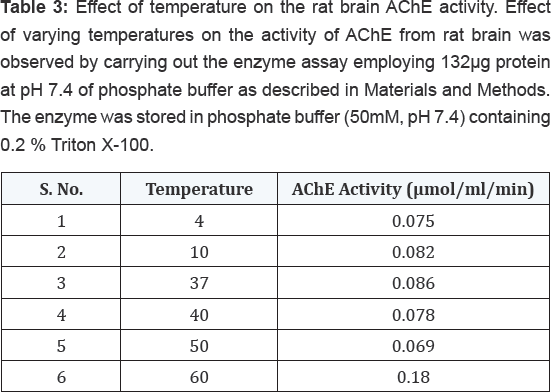
temperatures, it exhibited maximum activity at 370C followed by gradual loss in its activity after increasing temperature as shown in Table 3.
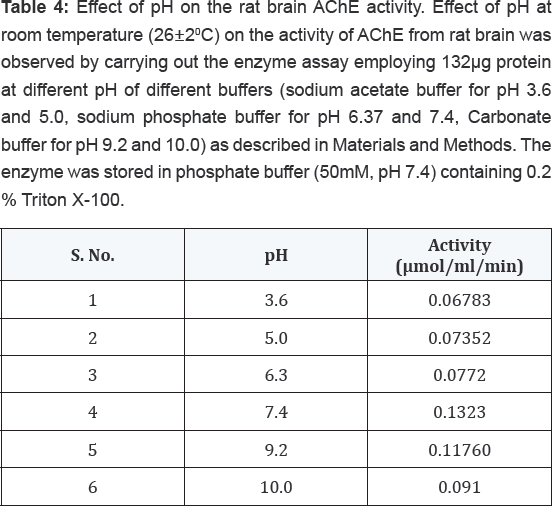
The enzyme was found to be optimally active at pH 7.4 when assayed
using buffers of different pH systems under standard assay conditions as
shown in Table 4. The buffers of higher pH values displayed inhibitory effect on enzyme activity.
Effect of substrate concentration on the activity of AChE from rat brain tissue
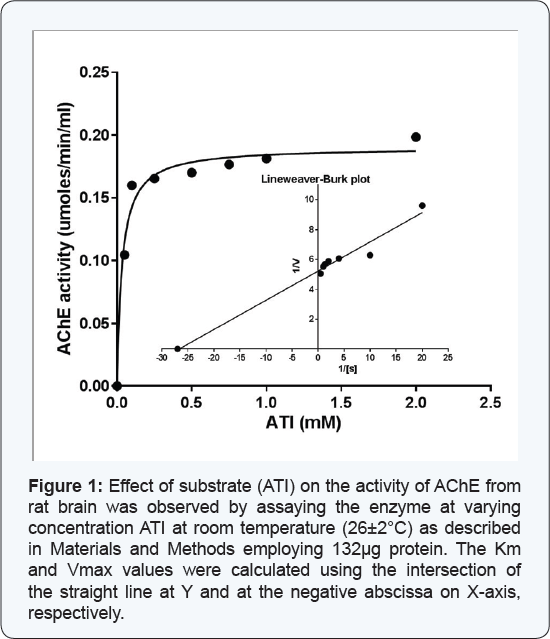
The enzyme (132μg) was assayed at varying concentrations of the substrate (ATI), at room temperature (26±20C).
The enzyme activity at corresponding substrate concentration displayed a
direct correlation and the results showed a hyperbolic curve (Figure 1). The Lineweaver Burk's double reciprocal plot of the data as shown in Figure 1 demonstrated a straight line which intersects at Y and negative abscissa of X-axes, from where the Vmax and Km values could be calculated; the values being 0.192μmoles ml-1min-1 and 0.037mM, respectively.
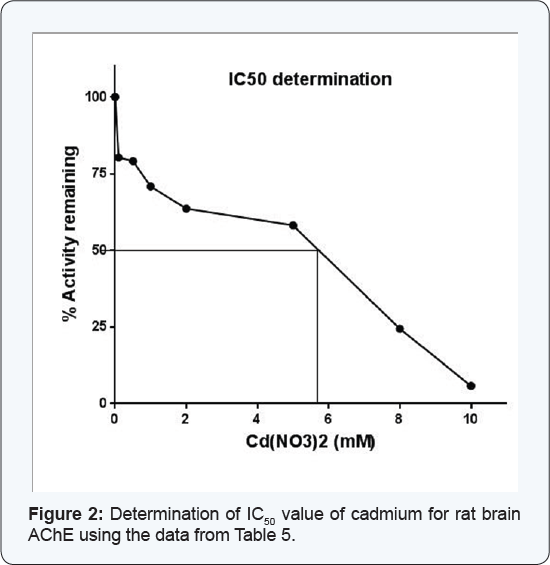
Effect of cadmium on the activity of AChE from rat brain tissue
The enzyme when assayed in presence of varying
concentrations of cadmium (0.05-10mM) displayed consistent decrease in
its activity (Table 5).
When this data was extrapolated using percent residual activity and the
cadmium concentrations on Y and X-axes, respectively, the IC50 value of this heavy metal for rat brain AChE could be determined, the value being 5.70mM (Figure 2).

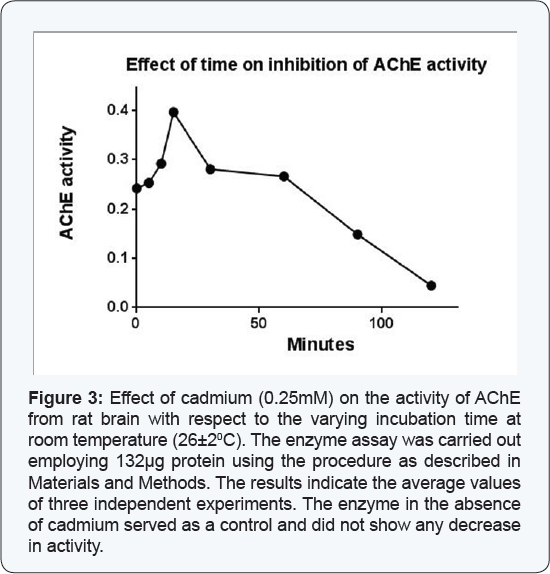
Effect of time on cadmium mediated inhibition of AChE activity from rat brain tissue
The effect of time on the cadmium mediated inhibition of the AChE activity from rat brain at room temperature (26±20C)
was monitored by assaying the enzyme (132μg) at different time
intervals in absence and presence of the cadmium (0.25mM). The enzyme
activity in the absence of cadmium served as a control. The results as
presented in Figure 3
demonstrated that the enzyme activity increased up to 15 min and after
that it decreased consistently with respect to the increasing incubation
time in presence of cadmium. The results from present study also
demonstrated that cadmium at a concentration of 0.25mM did not shown any
effect on its activity up to 15 min but it shows sharp inhibition of
the enzyme in a time dependent manner at 26±20C temperature. The enzyme activity remained about 50% after 77 min of incubation, i.e. the t1/2
time. However, the enzyme did not show any decrease in activity in the
absence of cadmium under similar experimental conditions.
Evaluation of mode of inhibition of AChE from rat brain by cadmium
The above experiments indicated that treatment of rat
brain AChE with cadmium resulted into decline in its activity. In order
to ascertain the mechanism of inhibition of AChE activity by
cadmium, the enzyme (132μg) was assayed at varying substrate
concentrations in absence and presence of cadmium (1.0mM). The data were
used to extrapolate Lineweaver Burk's double reciprocal plot, which
developed two straight lines originated from same point on X-axis and
intersecting at different points on Y axis (non-competitive inhibition).
The V max+I (Vmax value in presence of cadmium) and Ki
values were calculated using these points and found to be 0.069
μmoles/ml/min and 0.548mM, respectively. The results are shown in Figure 4.

Discussion
Before an enzyme can be characterized, it must be
extracted and solubilized from the selected tissue. Some of the enzymes
are membrane-bound and their solubilization has been shown to be
achieved by using organic solvents, detergents, proteolytic and
lipolytic enzymes [21].
It has been shown that EDTA and tetracaine increased the amount of
enzyme extracted from calf brain, and a preliminary studies showed that
these compounds had a similar effect on the extraction of enzyme from
mouse brain. The results from the present study demonstrated the
membrane bound form of rat brain AChE which could be easily solubilized
using Triton X- 100. In some other living systems also the membrane
bound nature of AChE has been indicated [22-24].
However, AChE has been shown to exist only in a membrane bound form in
the human erythrocytes and brain, electric eel, electric fish and
certain parasite helminthes [22,23].
The enzyme from these systems has been solubilized by applying both the
ionic as well as non-ionic detergents in the phosphate buffer [22]. The Km value for any enzyme is a measure of its affinity towards its substrate. In the present study, the Km
value for rat brain AChE was 37μM which was about three times lower
than that reported for the enzyme from human brain (107μM), and fetal
bovine serum (120 μM) [25]. The rat brain AChE displayed Km about 6times lower than that reported for the analogous enzyme from the human erythrocytes (225μM). The relatively lower Km value for rat brain AChE indicated its enhanced affinity to the substrate as compared to other mammalian systems [22].
The entry of cadmium in mammalian brain has been demonstrated. It is
therefore presumed that it may alter brain function. The results from
the present study displayed that cadmium caused strong inhibition of rat
brain AChE (IC50=5.7mM). These results indicate that cadmium
may act as an inducer of toxic stress on the neurotransmission system
of rat. In the present study, cadmium inhibited the activity of rat
brain AChE in noncompetitive manner when tested in vitro. These
results suggest the binding of cadmium at a different site on the enzyme
surface other than the active site. No such reports are available from
other workers to be used for the sake of the comparison.
Conclusion
The results from the present study indicated the
presence of membrane bound form of AChE in the rat brain which could be
solubilized employing a non-ionic detergent, Triton X-100. Cadmium
sharply inhibited this enzyme at low concentration indicating thereby
its strong neurotoxic potential to the mammals. Though the exact
mechanism of action of cadmium on brain AChE is not known but this study
presented evidence that this heavy metal may inhibit the enzyme in a
noncompetitive manner. Thus, the rat brain AChE may serve as a potential
biomarker of neurotoxicity induced by cadmium. The information obtained
from this study may be useful in proper risk management of cadmium
toxicity particularly in those who are occupationally engaged in cadmium
infested environment.
For more
details Open Access Journal of Toxicology (OAJT) please
click on: https://juniperpublishers.com/oajt/index.php
To read more…Full Text
in in Juniper Publishers click on https://juniperpublishers.com/oajt/OAJT.MS.ID.555553.php

Comments
Post a Comment