The Importance of Dose-Time-Response Relationships for Hazard Identification and Limitation of Animal Experiments- Juniper publisher
JUNIPER PUBLISHERS- Open Access Journal of Toxicology (OAJT)
Author: Henk A Tennekes
Introduction
Historian Heiko Stoff has recently sketched a fascinating controversy in the 1950's on chemical risk assessment [1]. Two renowned scientists in the Farbstoffkommission (Dye Committee) of the Deutsche Forschungsgemeinschaft
(DFG, German Research Community), pharmacologist Hermann Druckrey and
biochemist (and Nobel Prize winner) Adolf Butenandt, were advocates of a
preventive risk approach. This approach was largely determined by a
groundbreaking study conducted by Druckrey during the war years with the
carcinogenic dye 4-dimethylaminoazobenzene (also known as "butter
yellow") [2],
and Druckrey's un-intentional cooperation with the electrophysicist
Karl Küpfmüller in an American detention camp in Hammelburg, Bavaria [3].
The History of the Druckrey-Küpfmüller Equation
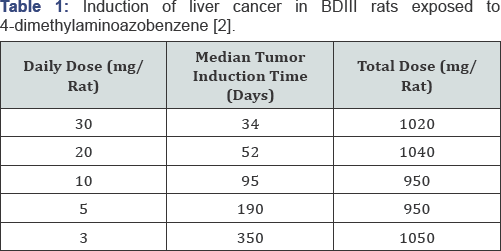
Druckrey demonstrated in 1943 that the carcinogenic
action of "butter yellow" was determined by the total dose, and
completely independent of the daily dose (Table 1). The product of daily dose D and exposure time (up to liver cancer manifestation) T was always the same:
- D. T = constant (1)
and found to be about 1000mg (1 gram) in the case of butter yellow (Table 1).
This dose-effect relationship is known as Haber's rule (or law) [4],
named after the German chemist Fritz Haber, who played a key role in
chemical warfare in the Great War. Haber's rule originally described the
relationship between gas concentration c and time to death t. The
smaller the c.t product, the higher the toxicity. Druckrey's observation
that Haber's rule also described the dose-response relationship of a
carcinogenic substance was remarkable in view of long latency periods.
Druckrey concluded from this study that the harmful effects of a
carcinogen were cumulative and that thresholds of toxicity for
carcinogens do not exist.
Druckrey and Kupfmuller also explained this
dose-response relationship theoretically with a mathematical analysis of
receptor kinetics, as shown in Table 2 [5].
It was assumed that the carcinogenic effect of butter yellow was due to
irreversible interactions with a specific receptor. We now know that
the receptor is DNA, and that cancer is the result of cumulative damage
to DNA, but that was not known in those years. However, Druckrey and
Kupfmuller also postulated that when the effect of receptor binding is
irreversible as well, effects would be amplified over time (Table 2). In 1956, when Peter Magee and John Barnes linked the carcinogenicity of dimethylnitrosamine to alkylation of nucleic acids [6],
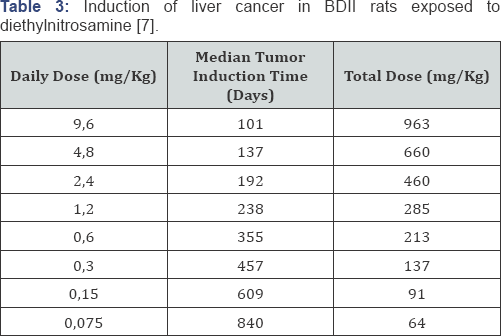
Druckrey took the initiative to investigate the dose-time relationship
of diethylnitrosamine (DENA) in rats to verify possible reinforcement of
effects by exposure time. After all, irreversible DNA alkylation
results in irreversible mutations, and nitrosamines therefore appeared
to be perfect model substances. This study confirmed potentiation of
carcinogenicity by time [7] which could be expressed as follows:
D. T n = constant (2)
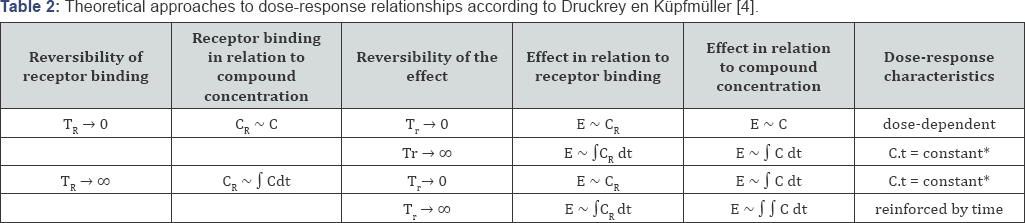
TR is the time constant for the reversibility of receptor binding.
Tr is the time constant for the reversibility of the effect.
C is the concentration of the compound at the site of interaction with the receptor.
CR is the concentration of bound receptors.
E is the effect.
* if C remains constant.
with a value ofthe time exponent n of 2.3. This
dose-response relationship is now known as the Druckrey-Kupfmuller
equation. The equation explains the harmful effects of low exposure
levels of a poison during prolonged exposure (Table 3).
The lower the daily dose, the lower the total dose required for the
damaging effect, even though the adverse effect occurs only after a long
exposure period.
Risk Prevention is Displaced by Risk Management
It is not surprising therefore that Druckrey and his
influential friend Butenandt became major advocates of risk prevention.
Only substances with a reversible mechanism of action and dose-dependent
toxicology (Table 2)
were acceptable in their eyes because safe exposure concentrations
below a threshold of toxicity could be defined. By contrast, substances
with an irreversible mechanism of action which followed equations (1)
and (2), had no threshold and exposure should, where possible, be
avoided.
However, in the 1960's, this approach was displaced
by the ADI (acceptable daily intake) concept, which defines an
acceptable level of exposure for a substance, independent of the
mechanism of action. The ADI concept was mainly propagated by French
professor René Truhaut [8]
and received a lot of support from the chemical industry, because it
was seen as a manageable concept for product development. This had major
consequences for toxicological research. The primary objective of
animal experiments was no longer clarification of the dose-response
relationship and the mechanism of action, but determination of the dose
that did not cause any harmful effect, in comparison to control animals,
the so-called No-Observed-Adverse-Effect Level (NOAEL). The NOAEL is
then divided by a safety factor, usually 100, to take account of
possible differences in sensitivity between experimental animals and
humans, and individual variation in sensitivity between humans. That
exposure level (NOAEL: 100) is then considered as the ADI, the
permissible daily human exposure. An exception was made for substances
with mutagenic (DNA-damaging) properties, which were not allowed unless
used to treat life-threatening diseases.
The Druckrey-Küpfmüller Equation is Generally Applicable
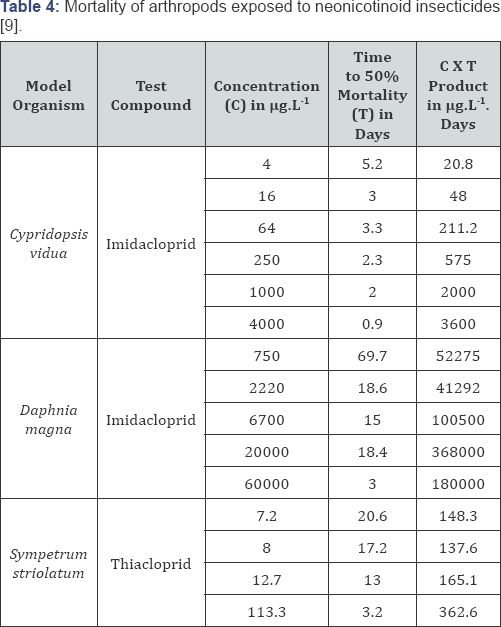
In 2009, Francisco Sanchez-Bayo showed that the
toxicity of the neonicotinoid insecticides imidacloprid and thiacloprid
for arthropods was not only dependent on the dose but also on the
duration of exposure [9].
Henk Tennekes, who was mentored by Hermann Druckrey in his time at the
German Cancer Center in Heidelberg, recognized the dose-response
relationships as Druckrey-Küpfmüller equations [10]. The lower the exposure concentration, the lower the total dose required for the damaging effect (Table 4).
The subsequent collaboration between Tennekes and Sanchez-Bayo provided
additional examples of substances with time-cumulative toxicity
(Cartap, Diphacinone, organic mercury) [11,12].
All of these substances cause irreversible receptor binding and
irreversible effects, and there are no indications for a threshold [13],
so that the ADI for these substances underestimate the actual risks. In
any case, it became clear that the theories of Druckrey and Küpfmüller
are generally applicable and that equations (1) and (2) are of
importance to the risk analysis of chemicals.
The Risks of an Irreversible Mechanism of Action are Underestimated
The widely used neonicotinoid insecticide
imidacloprid binds virtually irreversibly to nicotinic acetylcholine
receptors in the central nervous system of insects and causes
irreparable damage to nerve cells [14,15].
The substance is very slowly decomposed in the soil (half-life 200
days), and may leach into the groundwater, or run-off to surface water [16].
In water, the substance can only be degraded by ultraviolet light
(photolysis). In many areas of intensive agriculture, surface water is
contaminated with imidacloprid [16].
As a result, nontarget insects are exposed to an extremely toxic
substance for a long time, which can lead to massive insect mortality
and a break in the food chain [16].
Studies of the Universities of Utrecht and Nijmegen showed that the
pollution of surface water with imidacloprid quantitatively correlated
with decline of invertebrates and insects-dependent bird species [17,18].
Research in the National Park Dwingelderveld, The Netherlands, and in a
nature reserve in Krefeld, Germany, showed that, since the introduction
of imidacloprid in the mid-1990's, at least three-quarters of the
ground beetles and flying insects have disappeared [16,19]. The risks of imidacloprid have been completely underestimated, with catastrophic consequences for insects and insectivores.
Review of Risk Analysis is Urgently Required
Hermann Druckrey and Adolf Butenandt seem to have
defined the correct approach to risk analysis of chemicals in the
1950's, as the new insights of recent years show. The ADI concept of
René Truhaut is in any case unacceptable for substances with action
mechanisms described by Haber’s rule or the Druckrey- Küpfmüller
equation. Dose-response relationships are of much greater importance
than a NOAEL in an experimental experiment because irreversible effects
can be identified. In addition, dose- response relationships can make
accurate estimates of the risks in the real world. This may make risk
management more restrictive, but at least a lot safer. And there is
another important perspective. Dose-response relationships can make a
significant contribution to a strong reduction in the use of laboratory
animals (Table 4).
Analysis of Dose: Response Relationships Can Make Many Animal Studies Superfluou
The development of a new pesticide now costs almost $300 million [20],
and expenditure for international market authorization procedures have
risen exponentially in recent years. This development reflects the
increasing concern about harmful effects of pesticides on farmland
biodiversity. Imidacloprid is a case in point. The general applicability
of the theories of Druckrey and Küpfmüller means that analysis of dose-
response relationships can identify and eliminate hazardous substances
at an early stage of product development [21].
Dose- response studies can be performed in a short period of time, for
example with Daphnia magna (water fleas). In doing so, product
development is shifted to substances with dose-dependent toxicology (Table 2).
Long-term experimental experiments to determine a NOAEL are no longer
necessary, as exposure time has no effect. In combination with a
multitude of available in vitro studies
(http://www.oecd.org/env/ehs/testing/adopted-
testguidelines-toxicity-testing-3r-relevance.htm), this strategy can
make a major contribution to the implementation of the 3R Principles
(Replacement, Reduction and Refinement) that Russel & Burch first
described in 1959 to limit the use of experimental animals [22].
For more articles in JOJ Horticulture &
Arboriculture (JOJHA) please click on:
https://juniperpublishers.com/jojha/index.php
https://juniperpublishers.com/jojha/index.php

Comments
Post a Comment