Age Related Heavy Metal Accumulation in Sediment and Mangrove Roots in the Niger Delta Coastal Fringes, Nigeria
Open Access Journal of Toxicology
Introduction
Globally, marine and coastal ecosystems continue to be subjected to heavy metal pollution from municipal wastes, runoffs from agricultural and industrial sources [1-8] thereby, making coastal vegetations have a key function of trapping and storing pollutants [9-11]. The Niger Delta region remains a major concern in terms of heavy metal and hydrocarbon pollution due to increasing anthropogenic activities [12,13]. Accumulation of heavy metals in natural ecosystems is a threat to biodiversity and human health because of their persistence and toxicity [14-17] due to bioaccumulation and biomagnification effects [18,19]. The mangrove environment is very sensitive and vulnerable to pollution in view of its rich flora and fauna community. Mangroves are one of the most biologically important and productive ecosystems in the world [20]. MacFarlane reported that mangrove forests serve a key function of primary production in estuarine ecosystems and are an essential habitat for a wide variety of species such as birds, insects, mammals and reptiles [21,22].The proximity of mangroves to urban centers make them recipients of heavy metal contamination [23,24]. However, Mackey AP et al. [25] stated that mangroves are poor indicators of trace metals but found that large amounts of heavy metals are found in mangrove soils while few are found in plant tissues [26]. It is pertinent to state that even at low concentrations, heavy metals are poisonous due to bioaccumulation [27,28]. Mangrove forests are found in 118 countries around the globe with Nigeria’s Niger Delta area having the largest in Africa and fourth largest in the world in the order Indonesia>Brazil>Australia>Nigeria [29,30].
Biologically, six mangrove species make up these forests, three species in the family Rhizophoraceae (Rhizophora racemosa (red mangrove; tall), Rhizophora harrisonii (red mangrove; dwarf), Rhizophora mangle (red mangrove; dwarf)), and species in the family Avicenniaceae (white mangrove) and Combretaceae [31]. The mangrove forest of the Niger Delta is fast being depleted partly by Nypa palm invasion and wholly due to urbanization and industrialization. Accumulation of contaminants especially heavy metals occur in the roots but restricts its translocation to aerial portions of the plant hence less amount of heavy metals are found in the leaf compared to stem and root [26,32,33]. Heavy metals are not degradable but accumulate in plant tissues from soil which could cause long-term damage to plants particularly for mangrove soil with small grain size capable of accumulating such contaminants [34-36]. Most studies in the Niger Delta area had focused on the concentration of heavy metals in mangrove root and soil. The aim of this study was to assess the concentration of heavy metals (Pb, Cd and Ni) in the sediment and roots of two species of mangrove plants (Rhizophora, and Avicennia) in relation to the age of the plant root examined.
Materials and Methods
Site description
The study was conducted in mangrove forest areas on the coastal fringes of the Bonny estuary in the Niger Delta. The study areas included st.1: (Eagle island), St.2: (Bundu Ama) and St.3 (Borikiri) all in the southern Niger Delta region of Nigeria (Figure 1). The vegetation in the study area is mangrove with a mix of Avicennia, Rhizophora, Laguncularia and Nypa fruticans with Rhizophora as most dominant. The scanty mangrove trees in the area were irregularly disturbed with most at the fringes appearing under regenerative conditions of young age. Anthropogenic activities such as dredging, metal fabrication/maintenance works, oil servicing company activities, illegal oil bunkering activities and discharge of wastewater into nearby creeks characterized the study area.
Sample Collection
Mangrove Root Samples
Six (6) mangrove root samples were collected on a monthly basis for six months (December 2017 - May 2018). Samples for heavy metal determination were composited from triplicate samples to enhance wider coverage. Mangrove root (Rhizophora, Avicennia) samples were collected with sharp stainless-steel knife from three different sites studied. The plant roots were carefully collected from the part above the soil and divided into two parts. One part of each root sample was used to determine heavy metal concentration in roots while the corresponding part was used for age determination. The samples were properly labeled and immediately taken to the laboratory for analysis.
Sediment
Sediment samples were collected from the same stations as the root samples using soil/sediment sugar. Three spots around the root were sampled and composited. Samples were collected close to the root of the mangrove plant in order to correlate heavy metal content in both sediment and root samples. Sediment samples were put in properly labeled polythene bag and taken to the laboratory for analysis. All samples were preserved in mobile coolers while in transit.
Laboratory Analysis
Plant root samples were dried, grinded and digested with HCl/ HNO3 using the method of the American Society for Testing and Materials [37]. The concentration of heavy metals in plant root was determined with an Atomic Absorption Spectrophotometer (GB Avanta PM AAS, S/N A6600). Metal concentration was blank corrected and expressed as μgg-1 dry weight of sample for quality control.
Sediment
Samples were wrapped in properly labeled aluminum foils and put in ice coolers before taken to the laboratory for analysis. One (1g) of air-dried sediment was digested with Equia-Regia (mixture of HCI and HNO3 in the ratio of 3:1). The digested sediment samples were filtered with 20 ml of de-ionized water and the filtrates were stored in clean acid- washed and appropriately labeled 30 ml sample containers. Heavy metal analysis was done using Atomic Absorption Spectrophotometer.
Estimation of Age of Mangrove Root
A section of the mangrove root used for heavy metal analysis was air dried and surface polished to enable visualization of growth zones/ring bands. Triplicate samples were examined to obtain average age. Macroscopic and microscopic observations were made and ring-like formations (concentric circles) counted to estimate age of root. This is similar to methods used by other researchers to determine age of mangrove plants [38,39].
Data Analysis
Analysis of variance (ANOVA - General Linear Model) was used to test significant difference in metal concentrations across stations and also between the months of study. Tukey test was used for posting hoc analysis. Pearson correlation coefficient was used to determine relationship between metal concentration in root, sediment and age of plant root. The software Minitab 16 was used for the statistical analysis.
Results and Discussion
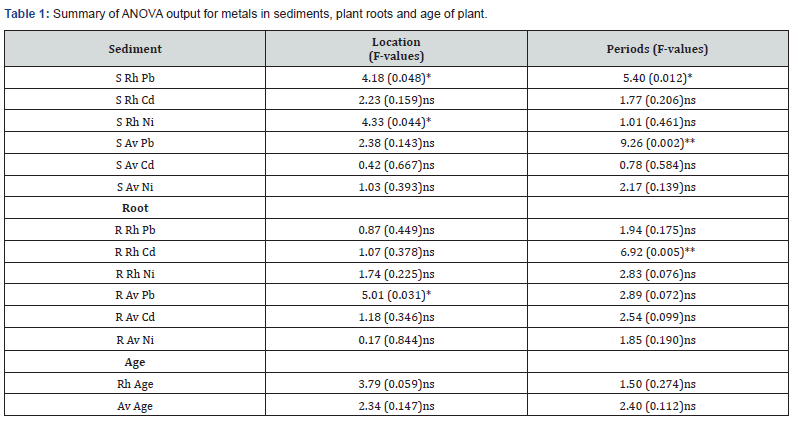
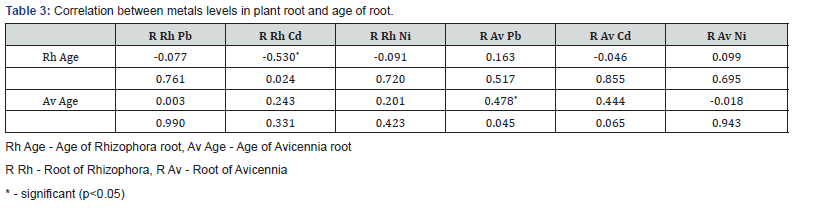
The concentration of heavy metals in sediments, roots of mangrove plants (Rhizophora-Rh and Avicennia- Av) and the age of plant roots examined is given in (Figure 2a-f). Table 1 gives a summary of the ANOVA output for metals in sediments, plant roots and age of roots. Table 2 has correlation of metal concentrations in sediments and plant roots while Table 3 presents that of metal concentrations in plant roots and the age of the plant root.


Heavy metals in Sediments
Both temporal and spatial variations were quite visible in terms of the variables examined. For purposes of clarity, trends and comparison, graphs were plotted to highlight spatial variations on monthly distinctions in line with discussions. First set of samples collected in December, only Ni was considerably observed in root and sediments while Pb and Cd were < 0.02 and <0.001 μgg-1 respectively across study stations. The concentration of Ni in sediments (<0.018 - 5.0 μgg-1) and those in root (<0.018 - 6.5 μgg-1) showed differences in the accumulation of the same heavy metal by different mangrove plants root within the same ecosystem. This implies that level of Ni observed in sediment did not transmit to a corresponding proportion in the root of the respective plants in the same month.
The sediments around the root of Rhizophora had elevated values of Ni compared to actual Ni concentration in the root. Interestingly, Avicennia roots bioaccumulated more of the heavy metal relative to the surrounding sediments. This is an implication for phytoremediation of such metal in polluted environments. This finding agrees with that of [40] who also recorded higher values (μgg-1) of Ni-33.12, Cd-0.33 and Pb-5.01 in the root of Avicennia compared to the respective values (27.42, 0.02 and 0.53) of the metals in sediments. The age of the plant roots also varied (2.3 - 4.2 years) as Avicennia root of higher age concentrated elevated amount of Ni unlike those of Rhizophora. The second set of samples in January also had metal levels in sediment generally higher than values in mangrove plant roots with St.1 and St.3 having higher values compared to St.2. But Ni (7.0 μgg-1) and Cd (0.6 μgg-1) were only observed in sediments at St.3 and St.1respectively while Pb in sediment (2.3 - 7.3 μgg-1) and root (1.2 - 7.3 μgg-1) were recorded in all stations examined. The levels of Pb obtained in this study were generally higher compared to those (0.34 mg/kg) for polluted soils and 0.001mg/kg for unpolluted mangrove soils in the Niger Delta [41] but Cd values in the sediment of this study were comparable to concentrations reported by [41]. Avicennia root also concentrated more of Pb relative to the surrounding sediment regardless of root age unlike Rhizophora suggesting a relationship with intake from the surrounding environment. However, Rhizophora with the highest age (4.3 years) at St.1 did not accumulate the highest metal but Avicennia at St.3 had the highest concentration of Pb (7.3 μg/g) at the mean age of 3.3 years. Age difference between periods of sampling was quite minimal. At the 3rd sampling in February, all three metals were recorded in all stations in the order Pb>Ni>Cd with St.3
Metal concentration in sediment was Pb (<0.02 - 7.6 μgg-1), Ni (<0.018 μgg-1), Cd (<0.001 μgg-1) while those in root were Pb (<0.02 - 8.7 μgg-1), Ni (<0.018 - 2.2 μgg-1) and Cd (<0.001 - 1.0 μgg-1). Plant roots accumulated more metals particularly Avicennia roots when compared to the root of Rhizophora and the surrounding sediments. Avicennia root with higher age generally accumulated more metals relative to Rhizophora roots with no clear pattern in relation to age of plant root. Increase in age with corresponding rise in metal concentration in the root was shown more in Avicennia plant compared to other mangrove plants examined. This result agrees with the findings of other researchers that Avicennia is a highly efficient plant for bioaccumulation of heavy metal contaminant [17,33,40,42]. In the month of March only Pb (1.2 μgg-1) was observed in sediment around the root of Avicennia at St.1 but the roots of the plants indicated higher levels of Pb, Ni and Cd compared to values in the surrounding sediment across the study sites. The oldest plant root (Av - 6.3yrs) however, did not accumulate the highest concentration of metal at specific locations. The preference for root was clearly demonstrated by Pb, Ni & Cd across study sites where such metals were detected in the root but not in the surrounding sediments. This pattern was also observed in April samples with values in sediment as follows Pb: <0.02 - 6.5 μgg-1, Ni: <0.018 - 4.2 μgg-1, Cd: <0.001μgg-1) and values in root (Pb: 1.3 - 6.2 μgg-1, Ni: 0.1 - 3.2 μgg-1, Cd: <0.001 - 0.4 μgg-1). The levels of Cd and Ni of this study corroborate the findings of Gbosidom VL, Obute GC and Tanee FBG who reported similar ranges within the Niger Delta mangrove (Rhizophora) but at variance in terms of Pb content [43]. However, researchers elswhere had reported Ni values (mg/kg) in sediments around Avicennia as 25.08, 54.12 and 1.9 - 7.7 [42,44,45]. The observed trend shows Avicennia roots indicating higher accumulation of metals compared to Rhizophora roots with St.3 having elevated levels of metals in plant roots and sediments. This is due to St.3 having more input of upland drainage and other anthropogenic activities compared to other sites.
The Pb content of Rhizophora at St.3 indicated a corresponding increase with the Pb content (6.2 μg/g) of the surrounding sediment but did not show a proportional increase with root age (2.7yrs). This implies that mangrove plant roots with lower age can also accumulate higher levels of heavy metal contaminants than older plant roots with respect to mangrove type. The heavy metals (Pb, Cd and Ni) content of both sediment and plant root in this study were lower compared to the findings of [46] who reported (sediment= Cd; 28.10, Pb:41.53, Ni:28.08 μgg-1 and root - Cd; 343.08, Pb:502.04, Ni:609 μgg-1) in a crude oil polluted area within the Niger Delta. The last set of samples in May examined also affirmed higher accumulation of heavy metals in Avicennia root than the surrounding sediments compared to values found in Rhizophora root. Metal concentrations in sediments and roots was in the order Pb>Ni>Cd with variations across sites studied, hence, Pb and Ni generally remained higher in concentration compared to Cd. In another study, Nazli MF et al. [47] reported higher concentrations of Pb (83.1±3.1) and Cd (0.8±0.5) in sediments above the values of this study but had Cd values (0.6) in mangrove plant roots was comparable to that of this study. The value of Pb (92.9) in the root of mangrove plant reported by is at variance with those of this study [47]. The age of the plant root also varied across sites but incremental concentrations of metals with increase in age of root was more apparent in Avicennia. However, one deviation occurred at St.2 where the root of Avicennia with the highest age (7.3yrs) did not accumulate the highest metal level, interplay of other environmental factors may be responsible for such difference.
The concentration of Pb in sediments surrounding the root of Rhizophora showed significant spatial and temporal variations (p<0.05). Post hoc analysis showed actual concentration to occur thus: location (St.3
The concentration of Pb in the root of Avicennia was also significantly different (p<0.05) between stations examined with post hoc analysis indicated as St.30.05) across study stations and months. Such differences suggested the discrete nature of the three stations in terms of their heavy metal content but in most cases with no clear seasonal pattern of significant variations. Such differences were mainly due to point and non-point sources of run-off and other anthropogenic activities including indiscriminate wastes dumps in the study area. The heavy metal concentrations obtained in this study were lower than critical concentrations recorded for soil/sediments and upper ranges in plants and also lower than values in European union guide for heavy metals in soil [48,49]. However, the metal values in this study were slightly within and higher in some cases than the standard values suggesting an area prone to heavy metal contamination particularly at St.1 & St.3 with drainage inputs and other anthropogenic activities [50]. S Rh- sediment around Rhizophora root, S Av Cd - sediment around Avicennia root, R Rh- Root of Rhizophora, R Av - Root of Avicennia, F-values outside parenthesis, P-values: in parenthesis, ns; not significant, ** : significant (p<0.01), *: significant (p<0.05).
Correlation (Heavy metals in sediment and root of plants)
Pearson correlation in Table 2 was used to determine the magnitude and direction of relationship between levels of heavy metals in sediment and those in plant roots since the variables in question are independent. Other significant correlations existed due to interaction of different heavy metals but fell outside our study interest. The level of Ni in sediments around Avicennia had significant positive correlation (p<0.01) with the level of the metal in the root of the plant. This implies an increase in Ni content of the surrounding sediment translated into an increase in the Ni content of the Avicennia plant root. The implication is that Avicennia plant accumulated more metals with corresponding increase in the environment, but this trend was different for the Rhizophora plant root. The higher concentration of metals in Avicennia sp root in this study also consents with the findings of who reported a value range of 0.6 - 5.5μg/g. in similar environments [51].
S Rh - Sediment around Rhizophora root, S Av - Sediment around Avicennia root
R Rh - Root of Rhizophora, R Av - Root of Avicennia
ns - not significant, ** - significant (p<0.01)
Correlation (heavy metals in plant roots and age of roots)
The strength and direction of linear relationship between the level of metal in the root of the plant and the age of the plant was also examined with Pearson correlation as given in (Table 3). The level of Cd in the root of Rhizophora had a significant (p<0.05) linear negative relationship with the age of the plant root while the concentration of Pb in the root of Avicennia had a strong positive correlation. This implies that as the age of the root of Rhizophora increased it absorbed less Cd and possibly other metals while increase in the age of the root of Avicennia correspondingly increased intake of Pb and likely other metals depending on metal interactions. The effective intake of heavy metals by the root of mangrove plants may be governed by the nature of the plant, availability of the metal in surrounding soil, metal interactions and other environmental factors.
Rh Age - Age of Rhizophora root, Av Age - Age of Avicennia root
R Rh - Root of Rhizophora, R Av - Root of Avicennia
* - significant (p<0.05)
Conclusion
Sediment and mangrove plant root samples were collected from three different stations in the southern region (Port Harcourt) of Nigeria for the analysis of heavy metals (Pb, Ni and Cd). The age of the plant roots were also examined with a view to establishing relationship between heavy metal levels in sediment, plant root and age of root. Results indicated spatial and temporal variations in the heavy metals accumulated in the roots and the sediment/soil surrounding plant roots examined. Heavy metal concentration both in sediment and plant root was generally in the order Pb>Ni>Cd with higher concentrations observed at St.3 followed by St.1 and St.2. due to the anthropogenic activities at the various stations. The study concluded that different plant roots contain different concentrations of heavy metals irrespective of the concentrations within their surrounding sediment. Avicennia concentrated more metals with increase in the metal content of the surrounding sediment but this relationship was not observed for Rhizophora root and sediment metal content. Though, there were variations in the age of the plants, there was no clear pattern that the plant root with the highest age also had highest accumulation of metal. Importantly, the root of Rhizophora showed that increase in age was met with reduced metal (Cd) concentration while Avicennia generally accumulated more heavy metal (Pb) with increase in root age. Observed discrepancies in heavy metal concentration with respect to study site was mainly human factor induced.
For more open access journal, please click on Juniper Publishers
For more Open Access journal of toxicology articles, please click on Open Access journal of toxicology articles
https://juniperpublishers.com/oajt/OAJT.MS.ID.555632.php


Comments
Post a Comment