Anti-Contractile Mechanism of Resveratrol in Non-Vascular Smooth Muscle Under α1-Adrenoceptor Stimulation involves IP3-Receptor, Protein Kinase-C and NADPH Oxidase
Open Access Journal of Toxicology
Introduction
Reactive oxygen species (ROS) are products of most metabolic reactions [1]. When their production exceeds the physiological antioxidant protection that cells can withstand, harmful and damaging effects occur, such as lipid peroxidation and DNA oxidation. This imbalance is responsible for several diseases such as cancer, cardiovascular diseases like atherosclerosis and hypertension, and several complications of Diabetes Mellitus [2]. The production of ROS, however, is not always harmful, as can be demonstrated by phagocytic cell actions against invading microorganisms [3], and by the physiological effects of nitric oxide (NO) as an important neurotransmitter and vasodilator [4,5].
Important mechanisms induce an influx of ions from the extracellular environment to the intracellular medium, directly or indirectly, through the production of ROS, which results in increased Ca2+ concentration in the cell cytoplasm [6,7].
Ca2+ mobilization is not only related to diseases and worrisome lesions, but also to muscle contraction, which will be covered in this article. Cytosolic calcium concentration ([Ca2+]c) increases triggering of the Ca2+-calmodulin complexation, which is essential for the muscle contraction due to activation via phosphorylation of myosin light chain (MLC) [8]. Smooth muscle contractions are substantially dependent on adrenergic stimulation, through α1-receptor.
Norepinephrine (NE), non-selectively, and Phenylephrine (PE), selectively, stimulate α1-adrenoceptors coupled to Gq protein, to produce second messengers. Such receptors are located primarily in vascular and non-vascular smooth muscle but have also been found in cardiomyocytes [9].
Through Gq protein activation, the phospholipase C (PLC) triggers the production of second messengers: inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) [10,11]. The IP3 interacts with receptors (RIP3) in the sarcoplasmic reticulum, the principal, but not the unique reservoir of intracellular calcium [12], releasing Ca2+ into the cytoplasm. DAG, in turn, activates protein kinase C (PKC), which leads to the opening of Ca2+ channels on the plasmatic membrane. Furthermore, DAG activates the enzyme NADPH oxidase located in the membrane to produce ROS [13].
The NADPH oxidase system is commonly recognized as the main source of ROS production in the vessel wall. Decreasing mRNA expression of NADPH oxidase [14] has been successfully studied by using atorvastatin, the synthetic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. Besides its well-known lipid-lowering effects, atorvastatin presents pleiotropic effects including anti-atherogenic anti-inflammatory actions, inhibition of the in vitro oxidation of LDL, and reduction in various oxidative stress markers [15,16].
Studies aiming to associate α1-adrenoceptors and ROS production are greatly benefited by preparations presenting sympathetic innervation and/or high populations of such receptors. Anococcygeus smooth muscle is a smooth muscle widely used as an essential tool for studying the mechanisms involving sympathetic activation. Its adrenergic innervation corresponds to 60% compared to other innervations and α1 is the primary receptor [17-19]. Furthermore, another advantage of the anococcygeus smooth muscle is its easy isolation and the presence of a long lineal structure with a thin layer of muscle cells, allowing for prompt diffusion of drugs and ions [11,20]. As retractor penis muscles, the anococcygeus is part of the erectile machinery in male rodents [21]. Concerning the potential clinical importance of anococcygeus smooth muscle and its relation to male genital apparatus, thus, it is a useful pharmacological and physiological tool to study issues such as benign prostate hypertrophy, etc [22]. Currently, α1- antagonists are commonly used to relax the smooth muscle in the prostate for treating benign prostate hypertrophy related to lower urinary tract symptoms [23,24].
Antioxidant substances are being investigated with the intent to minimize or even solve pathological effects triggered by ROS. Among these is Resveratrol (RESV), an agent that has been attracting researchers’ attention since the 80’s after the “French paradox”, which linked red wine consumption to the reduction of cardiovascular problems [25,26]. RESV inactivates free radicals [27-29] and decrease the activity of the contractile machinery of vascular [30,31] and non-vascular smooth muscle [32,33] by regulating the phosphorylation of MLC stimulated by PE. Nevertheless, many of these data were obtained from studies in vascular smooth muscle. Moreover, there are few studies of non-vascular smooth muscle in the literature and the correlation between RESV effects and α1-adrenoceptors stimulation.
It is believed that RESV may be able to prevent the action of ROS related to its activation by adrenergic stimulation. In this sense, RESV could lead to a reduction in contractile response in non-vascular smooth muscle. Thus, this study aimed to investigate the mechanism of RESV on the contractile reactivity in isolated rat anococcygeus muscle after α1-adrenergic stimulation.
Materials and Methods
Drugs
The following drugs were used: phenylephrine (PE), trans-resveratrol (RESV), prazosin, 2-aminoethoxydiphenyl borate (2-APB) [20] and 2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol- 3-yl)-maleimide (GF109203X) (Sigma-Aldrich, Inc., St. Louis, MO, USA), atorvastatin (ATV; Fagron, São Paulo-SP, Brazil), NaCl, KCl, KH2PO4, CaCl2, MgSO4, NaHCO3 and C6H12O6 (Lab Synth®, Diadema- SP, Brazil), isoflurane (AstraZeneca®, Cotia-SP, Brazil). Phenylephrine was dissolved in distilled water; resveratrol was dissolved in 70% v/v ethanol, and 2-APB was dissolved in methanol with further dilution in distilled water before use. Working concentrations of ethanol and methanol in the bath were <0.01% (v/v). Previous experiments showed that the solvents used had no effects on preparations at the applied concentrations.
Tissue preparation and isometric force measurement
This study was approved by the Ethics Committee of Animal Experiments of UNAERP-CEP/UNAERP number 019/2012. Male Wistar rats (200g) were anesthetized with isoflurane and killed by decapitation. Anococcygeus is a paired smooth muscle arising from the vertebral column to the ventral side of the colon. It comes from tendinous origins on the posterior sacral vertebrae and runs caudad around both sides of the rectum to unite on its ventral aspect.
As both anococcygeus smooth muscles were isolated [16] and dissected from each rat, the right muscles were used to test the drugs while the left ones were used in control experiments in which the respective drug was dissolved [34]. After isolating the pair of muscles, they were separated, and each one was carefully freed of connective tissue, tied at both ends by cotton thread ligatures and placed in 5mL organ baths, which were oxygenated (95% O2 and 5% CO2) and warmed (37 °C). The baths of Krebs’ physiological salt solution (PSS) had the following composition (in mmol/L): 118.0 NaCl, 4.7 KCl, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25.0 NaHCO3 and 2.0 C6H12O6 (pH 7.4). Muscle strips were connected to a force transducer (Scientific Instruments®, West Palm Beach-FL, EUA) set to a resting tension of 0.5g and allowed to equilibrate for 1 hour before the protocols. During the resting periods, the bath solution was replaced every 15 minutes. After 1 hour, muscle preparations were stimulated twice with 60mmol/L KCl-PSS (equimolar), to generate reproducible contractions and then washed out back to the resting tension.
Effect of RESV on the contractile response stimulated with pe
This set of experiment was designed to discover the minimal concentration of RESV would interfere with the potency or efficacy of the concentration-response curves of PE.
Muscle preparations were stimulated with increasing and cumulative concentrations of PE (1nmol/L-100μmol/L) before and after 20 minutes incubation with different concentrations of RESV (10pmol/L to 1mmol/L). Each concentration of RESV was tested in different preparations.
Effect of RESV on the contractile response stimulated with PE in the presence of PZ, 2-APB, GF109203x or ATV
Concentration-response curves were performed in anococcygeus muscles with increasing and cumulative concentrations of PE (1nmol/L-100μmol/L) before and after 20 minutes incubation with RESV 100μmol/L in the presence or absence of Prazosin (Pz, 10nmol/L-α1-adrenoceptor antagonist), 2-APB (100μmol/L-IP3 receptor antagonist), GF109203X (5μmol/L - PKC inhibitor) or Atorvastatin (ATV, 100μmol/L - NADPH oxidase antagonist).
This set of protocols was intended to investigate the mechanism involved in the cascade under α1-adrenoceptor activation, in which RESV could interfere.
Data presentation and statistical analysis
Data are expressed as mean ± SEM. Significant differences between two groups (p<0.05 or p<0.001) were determined by Student two-tailed t-test for paired data or by One-way ANOVA followed by Newman-Keuls post hoc. The cumulative concentration- response curves to PE allowed us to analyze the pharmacological parameters are the maximal effect (Emax, also referred to as efficacy) and potency (pD2 = -log EC50). Contraction values are presented as normalized data (percentage, %) of KCl contraction.
Results
RESV reduced the efficacy and the potency of PE
To determine whether ROS are involved in the activation of α1-adrenoceptor upon stimuli with PE, different concentrations of RESV (10pmol/L to 1mmol/L) were tested. As shown in Figure 1, incubation with RESV 100μmol/L reduced maximal contraction induced with PE (101.78 ± 1.13% vs. 69.39 ± 5.31%, n=6; p<0.001), and RESV 1mmol/L reduced efficacy (100.31 ± 0.77% vs 8.92 ± 4.95%, n=6; p<0,001) and potency (6.47 ± 0.05% vs 5.58 ± 0.27%, n=6; p<0.05) of contraction induced with PE. The results suggest that ROS sensitive to RESV are possibly derived from activation of α1-adrenoceptors with PE.
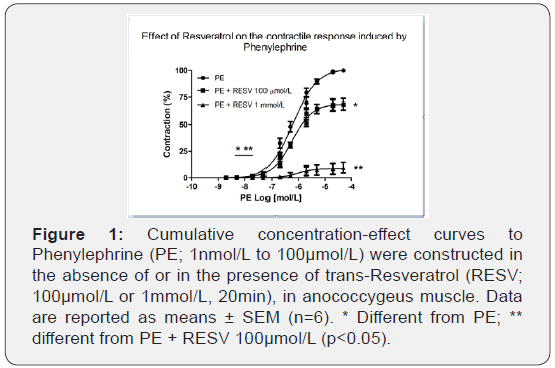
The concentrations of RESV that altered the PE-induced contractions were 100μmol/L and 1mmol/L. At lower concentrations, neither efficacy nor potency of the concentration-response curves of PE was changed (data not shown). The next experiments were done using 100μmol/L of RESV.
RESV in a combination of prazosin further decreased PE-induced contraction
It was examined whether ROS are directly involved in the activation of α1-adrenoceptor by using Pz, an α1-receptor antagonist. As shown in Figure 2, Pz 10nmol/L did not reduce efficacy but reduced the potency (6.27±0.09 vs. 5.35±0.09, n=6-7; p<0.001) of PE.
On the other hand, pre-treatment with Pz 10 nmol/L and RESV 100 μmol/L significantly reduced efficacy (100.06±0.68% vs. 55.78±4.37%, n=6-7; p<0.001) and the potency (6.13±0.06 vs. 5.44±0.05, n=6-7; p<0.001) of cumulative concentration-response curves to PE.
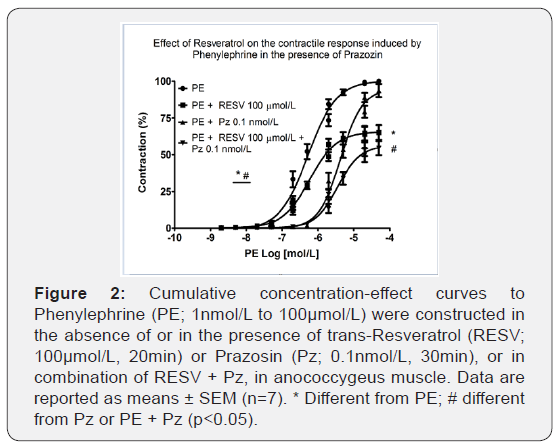
RESV in combination with 2-APB further decreased PE-induced contraction
To verify if ROS, sensitive to RESV, play a role in the intracellular contractile mechanisms after activation of α1-adrenoceptor we tested the contribution of IP3 receptors (RIP3) in this response using 2-APB, a RIP3 antagonist on the cumulative concentration- response curves to PE. Figure 3 shows that 2-APB 100μmol/L reduced both the efficacy (101.85±0.73% vs. 16.57±4.83%, n=6-7; p<0.001) and the potency (6.83 ± 0.27 vs. 5.76 ± 0.11, n=6-7; p<0.001) of PE. Furthermore, RESV 100μmol/L promoted an additional reduction to the inhibitory effect caused by 2-APB 100μmol/L. Therefore, the efficacy was practically abolished (99.3 ± 0.57% vs. 5.37±1.42%, n=6-7; p<0.001).
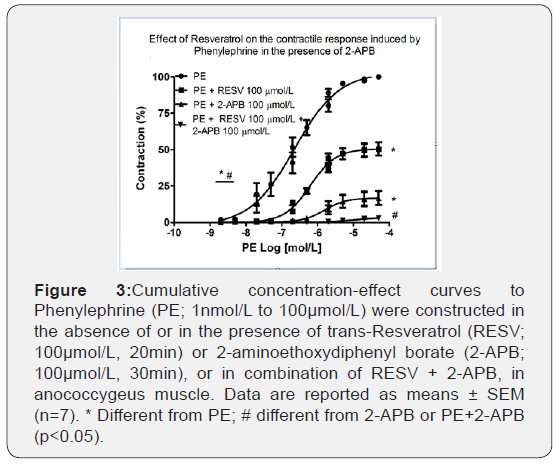
RESV in combination with GF109203X further decreased PE-induced contraction
The participation of ROS sensitive to RESV, under α1- adrenoceptor stimulation, and PKC activation was tested via GF109203X, a non-selective PKC inhibitor on the cumulative concentration-response curves to PE. As depicted in Figure 4, GF109203X 5μmol/L reduced the efficacy (100.21 ± 0.84% vs. 85.64 ± 4.02%, n=6-7; p<0.001) and the potency (6.46 ± 0.10 vs. 5.69 ± 0.08, n=6-7; p<0.001) of PE. The combination of RESV with GF109203X promoted a further reduction of the efficacy (100.21 ± 0.84% vs. 40.55 ± 7.36%, n=6-7; p<0.001). However, PE potency was not changed.
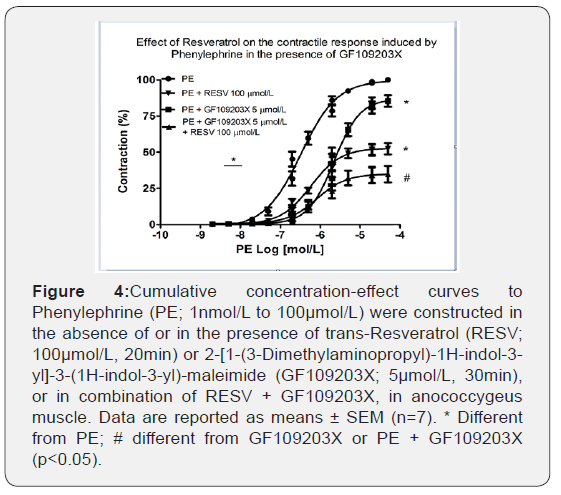
RESV in combination with ATV further decreased PE-induced contraction
The contribution of NADPH oxidase as a source of ROS, sensitive to RESV, was also investigated using ATV as a non-selective NADPH oxidase inhibitor. As seen in Figure 5, ATV 100 μmol/L did not reduce the efficacy but decreased the potency (6.37 ± 0.09 vs. 5.78 ± 0.05, n=6-7; p<0.001) of the cumulative concentration-response curves to PE. However, the addition of RESV 100μmol/L in a combination of ATV promoted a further decrease of the efficacy (99.96 ± 0.93% vs. 90.54 ± 4.29%, n=6-7; p<0.001) but did not change the potency of the contractions stimulated by PE, which were previously reduced by incubation with ATV alone.

Discussion
The present study is the first to demonstrate the anti-contractile effect of RESV, a compound with antioxidant activity, on the contractile machinery triggered by α1-adrenoceptors stimulation on anococcygeus smooth muscle. Different studies demonstrated ROS release promoted vasoconstriction [30,35]. Furthermore, it has been reported that activation of α1-adrenoceptor induces ROS releasing in vascular smooth muscles and some non-vascular smooth muscles [30]. Our data shows that RESV reduces both the potency and the efficacy of the cumulative concentration-response curves to PE in smooth muscle. Considering the already accepted antioxidant property of RESV [31], this finding confirms the hypothesis that contraction mediated by α1-adrenoceptor activation is positively associated with ROS activity in non-vascular smooth muscle.
It is known that superoxide increases the release of Ca2+ from intracellular stores and promotes an increase in its inflow to the intracellular environment [36]. Complementarily it is also known that ROS mediate α1-adrenoceptor-stimulated hypertrophy of vascular smooth muscle and cardiomyocytes, a long-term effect of catecholamines [9,37,38]. The contribution of ROS to the acute vasoconstrictor effect of α1-adrenoceptors was already characterized [30] for vascular smooth muscle.
Based on this observation, we tested the mechanism of RESV after the α1-adrenoceptor stimulus. Experimental protocols were designed to evaluate PE contractions in the presence of RESV, and the intracellular mechanisms activated after this stimulus, related to Ca2+ mobilization via IP3, DAG, the participation of PKC, and NADPH oxidase activation.
First, to evaluate the direct α1-adrenoceptors involvement, prazosin (Pz), a selective α1-adrenoceptor antagonist, was used alone or in combination with RESV so testing the contribution of ROS to PE-induced contractions. As a result, the concentration-response curves induced by PE in the presence of Pz presented a reduced potency, but not efficacy. Pz resulted in rightward shifts of the concentration-response curves to PE, with no depression of the maximum response. This finding is supported by literature [39]. Our original contribution is that, in the presence of RESV in a combination of Pz, PE stimulated contraction through α1- adrenoceptor on the anococcygeus smooth muscle isolated from rats was further reduced, suggesting the contribution of ROS, sensitive to RESV, on PE-induced contraction.
It is well known the activation of α1-adrenoceptor promotes an increase in [Ca2+]c leading to smooth muscle contraction and ROS play an essential role in this process [40]. The contribution of RIP3 on PE contraction was evaluated using the RIP3 antagonist, 2-APB [41]. To test the participation of ROS in the contraction via Ca2+ mobilization from internal stores, 2-APB was used alone and in combination with RESV. Our results identified a dynamic contribution of RIP3 on PE-induced contraction since the contraction was strongly reduced when the RIP3 was antagonized. Moreover, the presence of RESV in combination with 2-APB further decreased this contractile response, suggesting PE-induced contraction through ROS-dependent Ca2+ mobilization from sarcoplasmic reticulum on rat anococcygeus smooth muscle.
Slater et al. [42] demonstrated that RESV causes inhibition of protein kinase C (PKC) in endothelial cells. Considering that, besides Ca2+ from internal stores, the contraction induced by α1- adrenoceptor is partially dependent of increasing cytosolic Ca2+ concentration due to its influx in anococcygeus smooth muscle [12]; we have investigated the involvement of PKC on RESV effects. Similarly, to the IP3 receptor inhibition, PKC is also important to PE-induced contraction. Interestingly, RESV enhances the reduction of the PE-induced contraction in the presence of GF109203X, a non-selective PKC inhibitor, suggesting PE contraction triggers the production of ROS, and this is important to the activation of PKC.
The NADPH oxidases are the most important enzymes, whose role is to generate superoxide/ROS [43]. Since we observed ROS participated in the PE contractile response, the possible source of this ROS was investigated using atorvastatin (ATV) as a non-selective NADPH oxidase inhibitor [14,44,45]. According to Orallo et al. [46], the vasorelaxant activity of RESV enhancing nitric oxide signaling in the endothelium has been attributed to the inhibition of the vascular NADH/NADPH oxidase activity, leading to a reduction in basal superoxide production, and, consequently, decreased inactivation of nitric oxide. Our data shows that PE-induced contraction seemed to be partially dependent on NADPH oxidase activity in rat anococcygeus smooth muscle.
In our hands, in the presence of RESV and ATV, PE contraction was further reduced, pointing to a ROS-dependent activation of NADPH oxidase that contributes to PE-induced contraction in a non-vascular smooth muscle.
The main limitation of the present study is the lack of molecular and biochemistry studies. However, by pharmacological tools, it was possible to reach our aims.
Conclusion
The results support the view that ROS play a crucial role in the PE-induced contraction of rat anococcygeus smooth muscle. In addition, this contraction was dependent of α1-adrenoceptors, RIP3, PKC, and NADPH oxidase activation. Furthermore, the wellknown antioxidant RESV exerts an anti-contractile effect on PE contraction by mechanisms involving ROS-dependent RIP3, PKC and NADPH oxidase activation.

Figure 6 depicts, as a graphical conclusion, the proposed mechanism of RESV acting on the classical intracellular pathway triggered by alpha1 adrenoreceptor stimulation associated with H2O2 production by NADPH oxidase.
The authors thank the Brazilian National Research Council (Conselho Nacional de Pesquisa: CNPq) and University of Ribeirão Preto (UNAERP). The authors also thank Carla R. K. Antonietto for technical assistance.
For more open access journal, please click on Juniper Publishers
For more Open Access journal of toxicology articles, please click on Open Access journal of toxicology articles
https://juniperpublishers.com/oajt/OAJT.MS.ID.555627.ph


Comments
Post a Comment